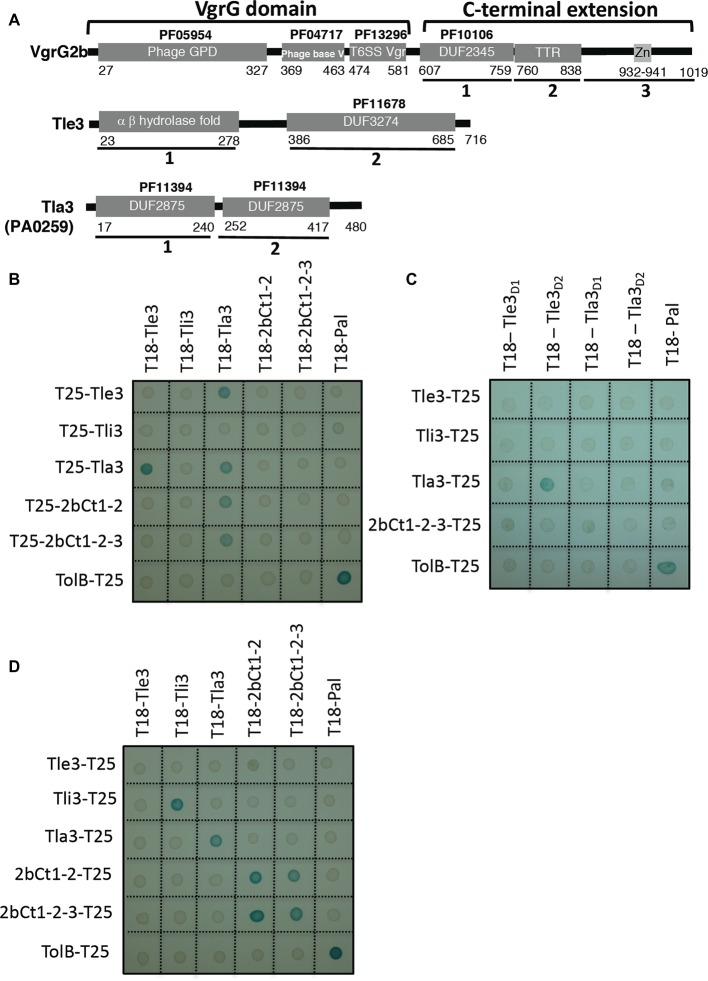
Figure 3.
(A) Tla3 (PA0259) interacts with the Tle3 toxin and with VgrG2b. Domain organization of VgrG2b, Tle3, and Tla3 (PA0259). The first 581 residues of VgrG2b carry the VgrG domain homologous to gp27 and gp5 phage tail proteins and consisting of three sub-domains. This is followed by the C-terminal extension, composed of a conserved domain of uncharacterized proteins (DUF2345, PF10106) (2bCt1), a TTR (transthyretin-like region) (2bCt2), and a putative zinc-dependent metallopeptidase pattern (LFIHEMTHVW signature, PS00142) (2bCt3). Tle3 architecture consists of an α,β hydrolase fold domain (Tle3D1) followed by a DUF3274 (Tle3D2), Tla3 of a tandem of DUF2875 (Tla3D1 and Tla3D2). (B–D) Bacterial two-hybrid assay. BTH101 reporter cells producing the indicated proteins or domains fused to the T18 or T25 domain of the Bordetella adenylate cyclase were spotted on X-gal indicator plates. The blue color of the colony reflects the interaction between the two proteins. TolB and Pal are two proteins known to interact but unrelated to the T6SS. The experiment was performed in triplicate and a representative result is shown.