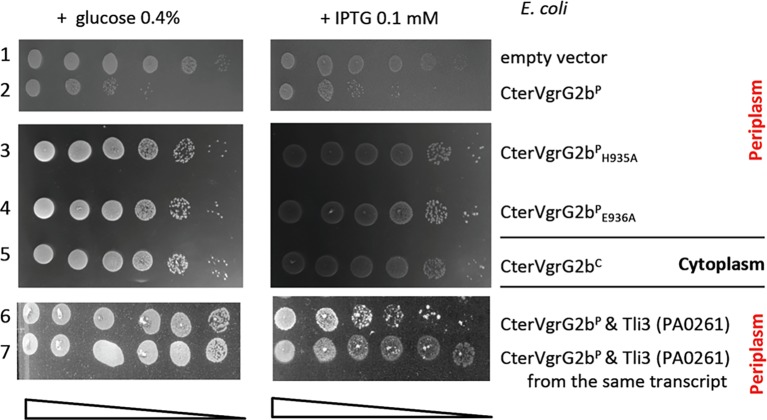
Figure 7.
The protease domain of VgrG2b is required for its antibacterial activity. Serial dilutions (from non-diluted to 10−5) of normalized cultures of E. coli BL21(DE3)pLys producing the wild-type C-terminal domain of VgrG2b in the cytoplasm, called CterVgrG2bC (from pBB43, a pETDuet-1 derivative, line 5) or in the periplasm, called CterVgrG2bP (from pBB44, a pET22b(+) derivative yielding a fusion of the C-terminal domain with a Sec signal peptide, line 2) or two variants CterVgr2bPH935A (from pBB45, line 3) and CterVgr2bPE936A (from pBB46, line 4) were spotted on LB agar plates supplemented (left panel) with 0.4% glucose or (right panel) with 0.1 mM IPTG. In line 6, the CterVgrG2bP (from pBB44) was coproduced with Tli3 (from pVT8) and in line 7, the CterVgrG2bP and Tli3 were coproduced from the same transcript (from pSBC110). Glucose and IPTG allow respectively repression and induction of the gene encoding the T7 RNA polymerase.