Abstract
Object
The association of age at menopause with endometrial cancer remains controversial. Therefore, we quantitatively summarized the evidence from observational studies with a meta-analysis.
Methods
We searched PubMed, Web of Science, Embase, Medline, Chinese National Knowledge Infrastructure (CNKI), and Wan Fang Med online up to March 2019, and all eligible case-control and cohort studies were included in the study. Pooled relative risks (RRs) with 95% confidence intervals (CIs) were calculated using the random-effects model. The dose-response relationship was assessed by restricted cubic spline model. The heterogeneity among studies was evaluated by I2. Metaregression was used to explore the potential sources of between-study heterogeneity. Egger's test was used to estimate publication bias.
Results
Eighteen articles including 957242 subjects with 4781 cases were included in the meta-analysis. The pooled RR (95%CI) of endometrial cancer for the highest versus the lowest age at menopause was 1.89 (95%CI: 1.58-2.26). For dose-response analysis, a nonlinear relationship was found between age at menopause and endometrial cancer, and the positive association became statistically significant when age at menopause was greater than 46.5 years old.
Conclusions
This meta-analysis suggested that age at menopause was positively associated with endometrial cancer. For women whose menopausal age over 46.5 years old, the risk of endometrial cancer increased with the age at menopause.
1. Introduction
Endometrial cancer is the most common gynecological tumor of the female [1]. Globally, endometrial cancer causes approximately 5% of cancer cases and over 2% of cancer deaths in women [2, 3]. It ranks the fourth most common malignant tumor in the female in developed countries [4]. Present studies indicate that genetic factors, anthropometric factors, lifestyle factors (e.g., tobacco smoking, alcohol drinking, physical activity, and usual diet), and clinically relevant diseases (e.g., diabetes, polycystic ovary) are related to endometrial cancer risk [5–14]. Besides, many reproductive factors that increase continuous stimulation of estrogen can also result in a higher risk of endometrial cancer, such as parity [15], age at menarche [16], oral contraceptive use [17], and breastfeeding [18].
Menopause as the terminus of women reproductive life is generally defined as a stop of menstruation for a consecutive year [19]. The age at menopause is of great clinical and public health significance [20]. Considering women with a later menopausal age have higher hormone levels and longer lifetime exposure to estrogens [21], age at menopause may be associated with many diseases. Studies had found that menopausal age was related to the risk of breast cancer and liver cancer [22–25]. However, the association between age at menopause and endometrial cancer is still controversial.
In order to explore the association between age at menopause and the risk of endometrial cancer, a large number of epidemiologic studies have been conducted [4, 26–42]. Among these studies, ten studies suggested a significant association between later age at menopause and an increased risk of endometrial cancer [4, 26, 30, 32–36, 38, 41], but the effect size in different studies was various, whereas no significant association was found in the other eight studies [27–29, 31, 37, 39, 40, 42]. Therefore, we conducted a meta-analysis to quantitatively evaluate the association between age at menopause and the risk of endometrial cancer risk.
2. Materials and Methods
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were adopted [43].
2.1. Search Strategy
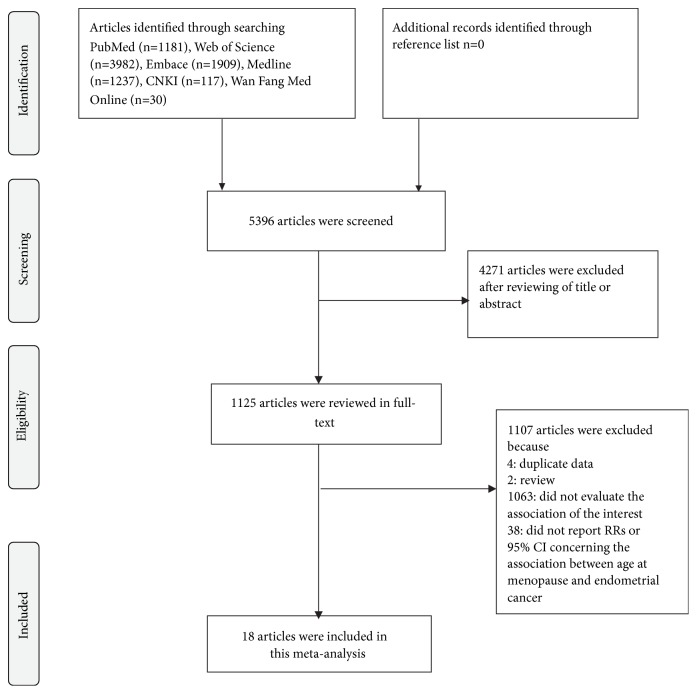
We used extended computer-based searches to obtain available studies published in English or Chinese from the databases of PubMed, Web of Science, Embase, Medline, Chinese National Knowledge Infrastructure (CNKI), and Wan Fang Med Online. The search terms used to search articles in this study were “age at menopause” (or “menopaus∗ age” or “the age of postmenopause” or “age at climacteric” or “climacteric age” or “pausimenia age”) and “endometrial cancer” (or “endometrial neoplasm” or “endometrial carcinoma” or “carcinoma of endometrium”). Relevant references within included studies were also manually searched. The detailed steps of the literature selection were shown in Figure 1.
Figure 1.
Flow chart of the selection of studies included in the meta-analysis.
2.2. Inclusion Criteria
If the article met the following characteristics, it would be included in our meta-analysis. (1) An observational study (cohort or case-control) was published as an original article. (2) The exposure of interest was categorized age at menopause. (3) The outcome of interest was endometrial cancer. (4) There was reported effect size (relative risk (RR) or odds ratio (OR) or hazard ratio (HR) or incidence rate ratio (IRR)) and 95% confidence interval (CI) for the association between age at menopause and endometrial cancer. (5) We selected the most recent study if data from the same population were used in multiple articles.
All identified studies were searched and reviewed carefully by two investigators (Yanjun Wu and Wenjun Sun). If the two investigators had different views on the same article, it would be settled by discussing with the third investigator (Dongfeng Zhang).
3. Data Abstraction
From each eligible article, we extracted the first author's name, country in which the study was performed, publication year, the type of study design, the follow-up duration of cohort study, age range or mean age at baseline, the number of cases, and controls in case-control studies as well as the person-year of cases in cohort studies. We also abstracted the information about age at menopause, RRs (we presented all results as RR for simplicity) with their 95%CIs for each category of age at menopause, and adjustment factors in each study, menopausal type, the definition of postmenopausal status, and the source of case information.
4. Quality Assessment
The Newcastle-Ottawa Scale (NOS) [44] was used to assess the quality of case-control studies and cohort studies included in this study. The scale was composed of three parts (selection, comparability, and outcome), with a maximum score of 9 stars.
4.1. Statistical Analysis
The pooled measure was calculated as the inverse variance-weighted mean of the natural logarithm of RR with corresponding 95% CI to assess the strength of association between age at menopause and the risk of endometrial cancer. Heterogeneity among studies was assessed by I2 proposed by Higgins and Thompson [45]. Metaregression was performed to explore potential sources of between-study heterogeneity [46]. The influence analysis with one study removed at a time was carried out to evaluate whether a single study could affect the results significantly. Publication bias was evaluated using Egger's test and funnel plot [47].
For dose-response analysis, a two-stage random-effects dose-response meta-analysis [48] was performed. In the first stage, a restricted cubic spline model with three knots at the 25th, 50th, and 75th centiles of the levels of age at menopause was estimated using generalized least square regression [49], taking into account the correlation within each set of published RRs [50]. Then the study-specific estimates were combined using the restricted maximum likelihood method in a multivariate random-effects meta-analysis [51]. A p value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to 0. The details of the statistical method have been described elsewhere [52].
All statistical analyses were performed with StataV.15.0 (Stata Corp., College Station, TX, USA). All reported probabilities (P values) were two-sided, and P values less than 0.05 were considered statistically significant.
5. Results
5.1. Study Selection
According to the search terms mentioned in the section of Materials and methods, we identified 1181 articles from PubMed, 3982 articles from Web of Science, 1909 articles from Embase, 1237 articles from Medline, 117 articles from CNKI, and 30 articles from Wan Fang Med Online. We excluded 4271 articles by reviewing the title and abstract. In the step of full-text article reviewing, we further excluded 1107 articles. Among them, four articles had the same population, two articles were reviews, 1063 articles failed to evaluate the association between menopausal age and endometrial cancer, and 38 articles did not have RRs (95% CIs) concerning the interests. Ultimately, 18 articles [4, 26–42] were included in this meta-analysis. The detailed steps of the literature selection were presented in Figure 1.
5.2. Quality Assessment
After using NOS to assess the quality of the 18 articles included in this study, the mean Newcastle-Ottawa score was 7.8 (range from 6 to 9) for case-control studies and 8.1 (range from 7 to 9) for cohort studies. The detailed results of the quality assessment were summarized in Tables S1 and S2.
5.3. Study Characteristics
In the 18 articles, nine articles were case-control studies [27–29, 31–33, 36, 38, 41] and nine articles were cohort studies [4, 26, 30, 34, 35, 37, 39, 40, 42]. With regard to continent where the study was conducted, five studies were conducted in Asian [27, 29, 32, 34, 40], four studies [4, 26, 36, 41] in Europe, and nine studies in North America. The endometrial cancer cases of most studies [4, 26, 27, 30–33, 35, 38–42] were identified from registry records (such as cancer registry) and five studies [28, 29, 34, 36, 37] were from hospital medical records. Six studies [27, 28, 32, 35, 37, 39] included women only with natural menopause, five studies [31, 33, 34, 41, 42] included women with both natural menopause and surgical menopause, and other seven studies [4, 26, 29, 30, 36, 38, 40] did not have relevant information. Information about the definition of postmenopausal status and the detailed characteristics of the included studies were shown in Table 1.
Table 1.
Characteristics of the included studies for age at menopause with risk of the endometrial cancer among postmenopausal women.
| Author | Country (year) | Age range or mean age | Study design (years of follow up) | Age at menopause | Case (control or person-year) | Participants | RR(95%CI) | Adjustment for covariates | Menopausal type and definition of postmenopausal status | Source of case information |
|---|---|---|---|---|---|---|---|---|---|---|
| Kvale G [26] | Norway (1988) |
27-69 | Cohort (20) |
≤45 | 16 | 62079 | 1 | Age, residential type, parity | NA | Cases were identified by records from the Cancer Registry of Norway |
| 46-47 | 9 | NA | ||||||||
| 48-49 | 20 | |||||||||
| 50-51 | 21 | |||||||||
| 52-53 | 15 | |||||||||
| ≥54 | 15 | 2.55(1.25-5.20) | ||||||||
|
| ||||||||||
| Shu XO [27] | China (1991) |
18-74 56.0/56.4 |
Case-control | ≤45 | 29 (44) | 404 | 1 | Age, number of pregnancies, weight | ① | Cases were identified by records from the Cancer Registry of Shanghai (1988-1990) |
| 46-48 | 25 (50) | 1.00(0.50-2.00) | ||||||||
| 49-50 | 67 (56) | 2.30(1.20-4.40) | ||||||||
| ≥51 | 73 (60) | 1.90(1.00-3.60) | ||||||||
|
| ||||||||||
| Brinton LA [28] | America (1992) |
20-74 59.2/58.0 |
Case-control | <45 | 39 (28) | 504 | 1 | Age, education, parity, recent weight, OC use, menopausal estrogen use | ① | Cases were identified by medical records |
| 45-49 | 89 (69) | 0.88(0.50-1.70) | ||||||||
| 50-54 | 133 (83) | 0.99(0.50-1.80) | ||||||||
| ≥55 | 36 (27) | 0.77(0.40-1.70) | ||||||||
|
| ||||||||||
| Hirose K [29] | Japan (1999) |
30-80 56.6/48.5 |
Case-control | ≤47 | 12 (1408) | 7849 | 1 | Age, BMI | NA | Cases were identified by medical records |
| 48-52 | 36 (4738) | 0.88(0.45-1.69) | ||||||||
| ≥53 | 24 (1631) | 1.50(0.75-3.03) | ||||||||
|
| ||||||||||
| Olson JE [30] | America (1999) |
55-69 | Cohort (10) |
<45 | 41 (30853) | 24848 | 1 | Age | NA | Cases were identified by records from the State Health Registry of Iowa |
| 45-49 | 61 (56522) | 0.80(0.60-1.20) | ||||||||
| 50-54 | 148 (106394) | 1.10(0.80-1.50) | ||||||||
| >55 | 82 (30987) | 2.00(1.40-2.90) | ||||||||
|
| ||||||||||
| Salazar-Martinez E [31] | Mexico (1999) |
57.1/54.6 |
Case-control | ≤40 | 7 (32) | 356 | 1 | Age, anovulatory index, smoking, diabetes mellitus, hypertension, physical activity, BMI | ② | Cases were from the gynecological oncology service and identified by biopsy-positive result. |
| 41-45 | 13 (61) | 1.10(0.34-3.70) | ||||||||
| 46-50 | 16 (88) | 1.00(0.34-3.30) | ||||||||
| ≥51 | 22 (117) | 1.20(0.42-3.60) | ||||||||
|
| ||||||||||
| Xu WH [32] |
China (2004) |
30-69 55.2/55.1 |
Case-control | <45 | 31 (66) | 1025 | 1 | Age, education, OC use, alcohol drinking, any cancer history among first degree relatives, BMI, ever having had a live birth and ever having had an induce abortion, age at menarche | ① Women were considered postmenopausal when they reported not having had any menses over the past 12 months |
Cases were identified by records from the Cancer Registry of Shanghai (1997-2001) |
| 45-49 | 192 (247) | 1.67(1.03-2.70) | ||||||||
| 50-54 | 220 (204) | 2.38(1.46-3.87) | ||||||||
| ≥55 | 48 (17) | 5.15(2.48-10.69) | ||||||||
|
| ||||||||||
| Trentham-Dietz A [33] | America (2006) |
40-79 62.9/63.3 |
Case-control | <50 | 177 (716) | 2464 | 1 | Age | ② |
Cases were identified by records from Wisconsin Cancer Reporting System |
| 50-55 | 239 (866) | 1.20(0.96-1.50) | ||||||||
| >55 | 134 (332) | 1.69(1.30-2.21) | ||||||||
|
| ||||||||||
| Wernli KJ [34] | China (2006) |
NA | Cohort (9) |
<45 | 4 (62227) | 267400 | 1 | Age, parity | ② Women were considered postmenopausal when they reported not having had any menses over the past 6 months. |
Cases were identified by medical record |
| 45-49 | 36 (310089) | 2.09(0.56-7.75) | ||||||||
| 50-54 | 81 (394730) | 3.73(1.03-13.47) | ||||||||
| ≥55 | 19 (38686) | 9.09(2.78-29.69) | ||||||||
|
| ||||||||||
| Setiawan VW [35] | America (2007) |
45-75 61.6 |
Cohort (7.3) |
<45 | NA | 46933 | 1 | Age, BMI, age at menarche, parity, OC use, smoking, diabetes, hypertension, family History of endometrial cancer, race |
① |
Cases were identified by records from Hawaii Tumor Registry |
| 45-49 | 1.27(0.88-1.84) | |||||||||
| 50-54 | 1.67(1.16-2.41) | |||||||||
| ≥55 | 1.79(1.15-2.78) | |||||||||
|
| ||||||||||
| Zucchetto A [36] | Italy (2009) |
18-79 60/61 |
Case-control | <50 | 97 (252) | 1077 | 1 | Period of interview, BMI, age at menarche, parity, OC use | NA | Cases were identified by medical records. |
| 50-54 | 193 (358) | 1.57(1.13-2.18) | ||||||||
| ≥55 | 64 (113) | 1.76(1.14-2.72) | ||||||||
|
| ||||||||||
| Dossus L [4] | Denmark (2010) | NA | Cohort (8.7) |
≤50 | 221 (535097) | 302618 | 1 | Age, BMI, physical activity, alcohol, diabetes, smoking, education | Women were considered postmenopausal when they reported not having had any menses over the past 12 months, or when they were older than 55 years. | Cases were identified by record linkage, health insurance records, cancer and pathology registries and active follow-up of study subjects. |
| 51-52 | 106 (170078) | 1.32(1.04-1.68) | ||||||||
| 53-55 | 118 (156492) | 1.49(1.18-1.89) | ||||||||
| >55 | 53 (43212) | 2.20(1.61-3.01) | ||||||||
| Karageorgi S [37] | America (2010) |
30-50 41.8 |
Cohort (19.3) |
<45 | 41 (71578) | 121700 | 1 | Age, parity, age at first birth, age at last birth, OC use, PMH use, BMI, smoking, diabetes, family history of endometrial cancer, age at menarche | ① |
Cases were identified by medical records. |
| 45-49 | 141 (310321) | 0.82(0.57-1.18) | ||||||||
| 50-54 | 386 (521943) | 1.22(0.81-1.85) | ||||||||
| ≥55 | 68 (77867) | 1.25(0.78-2.01) | ||||||||
|
| ||||||||||
| Amankwah EK [38] | Canada (2013) |
40-79 62.1/62.6 |
Case-control | <50 | 127 (297) | 1103 | 1 | Age, residential type, smoking, BMI, hypertension | Postmenopausal status was defined as age ≥60 years, or self-identified as postmenopausal with the last menstrual period ≥12 months, or 50 to 59 years and using menopausal hormones for ≥2 years. | Cases were identified by records from Alberta Cancer Registry |
| 50-54 | 176 (348) | 1.23(0.92-1.64) | ||||||||
| ≥55 | 58 (97) | 1.55(1.03-2.33) | ||||||||
|
| ||||||||||
| Dashti SG [39] | America (2015) |
18-86 40 |
Cohort (15) |
<50 | 5 (3357) | 1128 | 1 | Country, education, ascertainment, age at menarche, parity, PMH use | ① Women were considered postmenopausal when they reported not having had any menses over the past 12 months |
Cases were identified by records from Colon Cancer Family Registry |
| ≥50 | 14 (4375) | 1.64(0.53-5.05) | ||||||||
|
| ||||||||||
| Jung KJ [40] | Korea (2016) |
55.6 | Cohort (12.1) |
≤45 | 5 | 66466 | 1 | Age, smoking, age at menarche, SBP, TC, HDL cholesterol, diabetes, exercise, exogenous hormone use, health insurance premium | Women were considered postmenopausal when they reported not having had any menses over the past 12 months | Cases were identified by records from National Health Insurance Service in Korea |
| 46-48 | 3 | 0.80(0.18-3.57) | ||||||||
| 49-51 | 12 | 2.68(0.82-8.78) | ||||||||
| ≥52 | 11 | 3.06(0.89-10.47) | ||||||||
|
| ||||||||||
| Yang HP [41] | Poland (2016) |
20-74 | Case-control | <45 | 33 (110) | 1 | Age, study site, age at menarche, oral contraceptive use, parity, LOC | ② |
Cases were identified by records from Polish Cancer Study | |
| 45-49 | 111 (355) | 1.44(0.83-2.48) | ||||||||
| 50-54 | 252 (579) | 2.71(1.38-5.35) | ||||||||
| ≥55 | 114 (179) | 3.59(1.62-7.96) | ||||||||
|
| ||||||||||
| Sponholtz TR [42] | America (2017) |
21-69 36.6 |
Cohort (18) |
<45 | 12 (22960) | 47555 | 1 | Age, study peroid, education, marital status, age at menarche, parity, menopausal status, OC use, estrogen-only female menopausal hormone use, estrogen plus progestin female menopausal hormone use, smoking status, BMI, physical activity | ② Women were considered postmenopausal when they reported not having had any menses over the past 12 months, bilateral oophorectomy, or when they were ≥57 years of age and whose menopausal status was obscured due to hormone use. |
Cases were identified by record linkage from state cancer registries |
| 45-49 | 38 (46155) | 1.45(0.73-2.89) | ||||||||
| 50-54 | 64 (64053) | 1.32(0.67-2.56) | ||||||||
| ≥55 | 28 (19161) | 1.45(0.70-3.02) | ||||||||
RR, relative risk; CI, confidence interval; NA, not available; BMI, body mass index; OC, oral contraceptive; PMH, postmenopausal hormone use; SBP, systolic blood pressure; TC, total cholesterol; HDL, high density lipoprotein; LOC, lifetime number of ovulatory cycles. ①, among women only with natural menopause; ②, among women with natural menopause and surgical menopause
5.4. Association between Age at Menopause and Endometrial Cancer
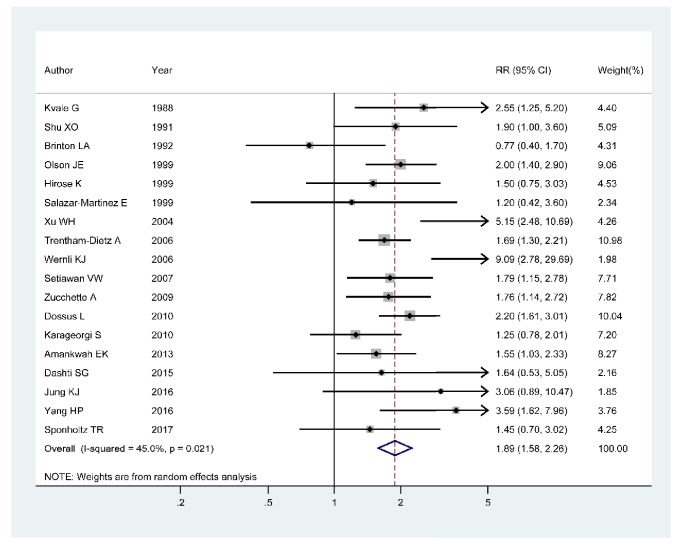
The association between age at menopause and endometrial cancer was evaluated in 18 articles [4, 26–42] with 957242 participants and 4781 cases. We could observe a statistically positive association between age at menopause and the risk of endometrial cancer in 10 articles [4, 26, 30, 32–36, 38, 41] of them, whereas the other eight studies [27–29, 31, 37, 39, 40, 42] showed no obvious association. The pooled RR of the risk of endometrial cancer for the highest versus the lowest age at menopause was 1.89 (95%CI 1.58–2.26; I2 = 45.0%, Pfor heterogeneity=0.021) (Figure 2).
Figure 2.
Forest plot of age at menopause and the risk of endometrial cancer. The size of gray box is positively proportional to the weight assigned to each study, and horizontal lines represent the 95% confidence intervals.
In the dose-response analysis of 15 articles [4, 27–34, 36–38, 40–42], a nonlinear association between age at menopause and endometrial cancer was found (Pnonlinearity < 0.01). The positive association became significant when age at menopause was greater than 46.5 years old. The RRs (95% CIs) of endometrial cancer risk were 1.04 (1.03-1.06), 1.17 (1.14-1.20), 1.57 (1.45-1.71), and 2.08 (1.80-2.39) for 47, 50, 54, and 57 years old of age at menopause, respectively (Figure 3).
Figure 3.
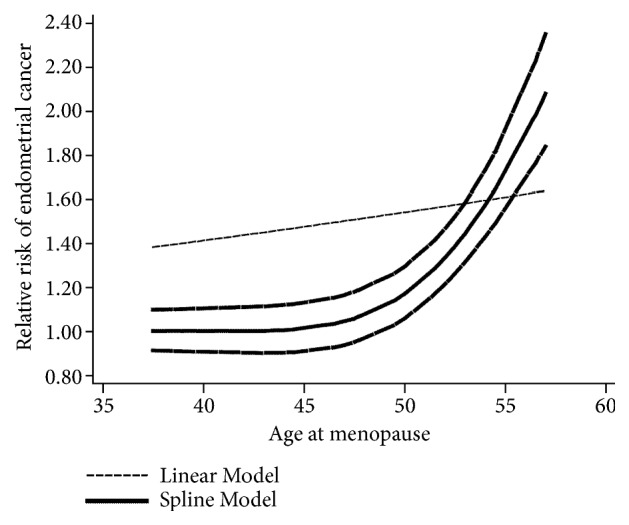
The dose-response analysis between age at menopause and the risk of endometrial cancer with restricted cubic splines in a multivariate random-effects dose-response model. The solid line and the long dash line represent the estimated relative risks and its 95% CIs. Short dash line represents the linear relationship.
5.5. Subgroup Analysis
In the subgroup analysis by continent where the study was conducted, the pooled RRs (95%CIs) were 1.60 (1.37–1.86), 3.06 (1.64–5.72), and 2.19 (1.74–2.75) for North America, Asia, and Europe, respectively. In the subgroup analysis by study design, the pooled RRs (95%CIs) for case-control studies and cohort studies were 1.80 (1.36–2.38) and 1.98 (1.56–2.52), separately. In the subgroup analysis performed according to menopausal type, the pooled RRs (95%CIs) were 1.72 (1.09-2.71), 2.25 (1.31-3.85), and 1.94 (1.63-2.30) for studies among natural menopausal women, studies among both natural menopause and surgical menopause women, and studies without relevant information, respectively. In the subgroup analysis by reference group of menopausal age, the pooled RR (95%CI) was 2.06 (1.51-2.81) for studies that used age at menopause ≤45 (<45 or ≤ 45 or ≤40) as reference group and the pooled RR (95%CI) was 1.79 (1.52-2.10) for studies that used age at menopause ≤50 (<50 or ≤ 50 or ≤47) as the reference group. In the subgroup by the Newcastle-Ottawa score, the pooled RRs (95%CIs) were 1.67 (1.12-2.49) and 1.98 (1.66-2.37) for studies with a score of 6 or 7 and studies with a score of 8 or 9. The detailed results of subgroup analysis were summarized in Table 2.
Table 2.
Summary of relative risks (RRs) for association of age at menopause with risk of the endometrial cancer.
| Stratification | Number of studies | RR (95% CI) | I 2,% | P for heterogeneity |
|---|---|---|---|---|
| All studies | 18 | 1.89 (1.58-2.26) | 45.0% | 0.021 |
| Continent | ||||
| North America | 9 | 1.60 (1.37-1.86) | 0.0% | 0.516 |
| Asia | 5 | 3.06 (1.64-5.72) | 63.5% | 0.027 |
| Europe | 4 | 2.19 (1.74-2.75) | 0.0% | 0.453 |
| Study Design | ||||
| Case-control study | 9 | 1.80 (1.36-2.38) | 54.5% | 0.024 |
| Cohort study | 9 | 1.98 (1.56-2.52) | 35.4% | 0.135 |
| Menopausal type | ||||
| Natural menopause | 6 | 1.72 (1.09-2.71) | 67.1% | 0.009 |
| Natural menopause and surgical menopause | 5 | 2.25 (1.31-3.85) | 64.0% | 0.025 |
| NA | 7 | 1.94 (1.63-2.30) | 0.0% | 0.729 |
| Reference group of menopausal age | ||||
| ≤45 (<45 or ≤45 or ≤40) | 12 | 2.06 (1.51-2.81) | 60.6% | 0.003 |
| ≤50 (<50 or ≤50 or ≤47) | 6 | 1.79 (1.52-2.10) | 0.0% | 0.761 |
| The Newcastle-Ottawa score | ||||
| 6 or 7 | 5 | 1.59 (1.18-2.15) | 27.1% | 0.241 |
| 8 or 9 | 13 | 2.05 (1.64-2.56) | 50.8% | 0.018 |
RR, relative risk; CI, confidence interval; BMI, body mass index; PMH, postmenopausal hormone
5.6. Meta-Regression
Multivariable metaregression with the covariates of publication year (p = 0.727), study design (p = 0.623), mean age of study participants (p = 0.538), menopausal type (p = 0.621), reference group of menopausal age (p = 0.554), and the Newcastle-Ottawa score (p = 0.362) showed that these covariates had no significant impact on the heterogeneity. But continent where the study was conducted (p = 0.014) was found to have an influence on the between-study heterogeneity.
5.7. Influence Analysis and Publication Bias
No study had excessive influence on the pooled RR for the highest versus the lowest age at menopause in the influence analysis. No evidence of significant small-study effect for the analyses was found by the visual inspection of the funnel plot and Egger's test (p=0.373). The results of the funnel plot were shown in Figure 4.
Figure 4.
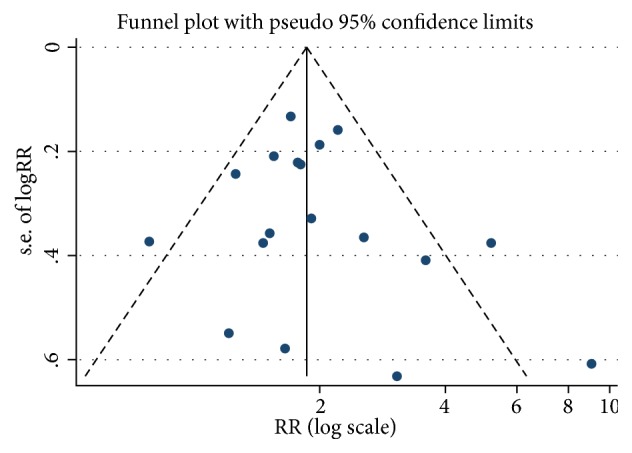
Funnel plot with pseudo 95% confidence limits for the analysis of age at menopause and risk of endometrial cancer (RR, relative risk).
6. Discussion
The average menopausal age for women varied among region [53]. Studies also showed that overweight, later age at menarche, and higher parity were associated with later menopausal age [54, 55]. In our study, we found that a later menopausal age was associated with an increased risk of endometrial cancer. In dose-analysis, a nonlinear relationship was found between age at menopause and endometrial cancer, and the positive association became statistically significant when age at menopause was greater than 46.5 years old. The positive association remained in subgroup analysis by continent, menopausal type, the reference group of menopausal age, and Newcastle-Ottawa score. Subgroup analysis especially by study design showed the positive association between age at menopause and endometrial cancer for cohort studies.
The mechanisms of higher endometrial cancer risk caused by later age at menopause are still equivocal. The hypothesis of “estrogen unopposed by progesterone” has been proposed as an important etiological factor [56, 57], which suggests that high levels of biological estrogens not counterbalanced by progesterone can result in a higher risk of endometrial cancer through increasing the mitotic activity of endometrial cells. Because the high-level female hormone can increase the probability of DNA-damaging (such as mitotic activity, DNA replication, and somatic mutations) and then become a fixed mutation [58, 59], people with a later menopausal age have higher hormone levels and longer time exposure to estrogens before menopause [21, 56]. In addition, later menopause might increase the risk of endometrial cancer by increasing the rate of spontaneous and environmentally induced mutations in endometrial stem cells [58]. Moreover, progesterone deficiency associated with anovulatory cycles is common in people having a later menopausal age, which may also contribute to endometrial cancer risk [60].
The issue of between-study heterogeneity should be paid particular attention in meta-analyses [61], and investigating the potential sources of between-study heterogeneity is necessary. The result of this meta-analysis showed moderate between-study heterogeneity in the analyses of age at menopause and risk of endometrial cancer. Multivariable metaregression with covariates of publication year, the continent where the study was conducted, study design, the mean age of study participants, menopausal type, reference group of menopausal age, and the Newcastle-Ottawa score was carried out to explore the potentially important source of heterogeneity. Among these covariates, just continent where the study was conducted was found to have a contribution to the between-study heterogeneity. After the subgroup analysis by continent, the positive association still remained in North America, Asia, and Europe and the pooled RRs were 1.60 (95% CI 1.37–1.86; I2= 0.0% Pfor heterogeneity= 0.516), 3.06 (95% CI 1.64–5.72; I2 = 63.5% Pfor heterogeneity= 0.027), and 2.19 (95% CI 1.74–2.75; I2 = 0.0% Pfor heterogeneity = 0.453), respectively.
The study has many virtues. First, compared with the original individual study, our meta-analysis has a large number of included participants, which can make the results more precise and more reliable. Second, the quality of the included articles was relatively high with Newcastle-Ottawa score ranging from 6 to 9. Third, the results are still meaningful after subgroup analysis by continent, menopausal type, the reference group of menopausal age, and Newcastle-Ottawa score, and subgroup analysis by study design also showed the positive association between age at menopause and endometrial cancer in cohort studies. Finally, we conducted a dose-response analysis to explore the association between age at menopause and endometrial cancer quantitatively.
However, there are also several potential limitations in our study. First, the adjusted confounders are different among studies and the residual confounding could not be eliminated thoroughly. Second, the age range of participant is disparate in every study and years of follow-up in cohort studies are diverse. Finally, the definition of postmenopausal status and the stratification of age varied among studies, which might affect the result.
7. Conclusions
Results from this meta-analysis indicated that age at menopause was positively associated with the risk of endometrial cancer. When the menopausal age of women exceeded 46.5 years, the risk of endometrial cancer increased with her menopausal age. For these women, they should develop good lifestyles to reduce the risk of endometrial cancer. More well-designed prospective studies are needed to confirm the association in the future.
Acknowledgments
Thanks are due to all the authors of included paper.
Disclosure
Ms Yanjun Wu and Dr Wenjun Sun are co–first authors.
Conflicts of Interest
We declare that we have no conflicts of financial and commercial interest.
Supplementary Materials
Table S1. Quality assessment of included case-control studies. Table S2. Quality assessment of included cohort studies.
References
- 1.Braun M. M., Overbeek-Wager E. A., Grumbo R. J. Diagnosis and management of endometrial cancer. American Family Physician. 2016;93(6):468–474. [PubMed] [Google Scholar]
- 2.Clarke M. A., Long B. J., Del Mar Morillo A., Arbyn M., Bakkum-Gamez J. N., Wentzensen N. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Internal Medicine. 2018;178(9):1210–1222. doi: 10.1001/jamainternmed.2018.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. International Journal of Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Dossus L., Allen N., Kaaks R., et al. Reproductive risk factors and endometrial cancer: the european prospective investigation into cancer and nutrition. International Journal of Cancer. 2010;127:442–451. doi: 10.1002/ijc.25050. [DOI] [PubMed] [Google Scholar]
- 5.Pabalan N., Pineda M. R., Jarjanazi H., Christofolini D. M., Barbosa C. P., Bianco B. Association of the +331G/A progesterone receptor gene (PgR) polymorphism with risk of endometrial cancer in caucasian women: a meta-analysis. Archives of Gynecology and Obstetrics. 2015;291(1):115–122. doi: 10.1007/s00404-014-3344-z. [DOI] [PubMed] [Google Scholar]
- 6.Zhou B., Yang L., Sun Q., et al. Cigarette smoking and the risk of endometrial cancer: a meta-analysis. American Journal of Medicine. 2008;121(6):501.e3–508.e3. doi: 10.1016/j.amjmed.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Schmid D., Behrens G., Keimling M., Jochem C., Ricci C., Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. European Journal of Epidemiology. 2015;30(5):397–412. doi: 10.1007/s10654-015-0017-6. [DOI] [PubMed] [Google Scholar]
- 8.Liao C., Zhang D., Mungo C., Andrew Tompkins D., Zeidan A. M. Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? a systematic review and meta-analysis of cohort studies. Gynecologic Oncology. 2014;135(1):163–171. doi: 10.1016/j.ygyno.2014.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aune D., Rosenblatt D. A. N., Chan D. S. M., et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Annals of Oncology : Official Journal of the European Society for Medical Oncology. 2015;26(8):1635–1648. doi: 10.1093/annonc/mdv142. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q., Guo P., Li H., Chen X.-D. Does alcohol consumption modify the risk of endometrial cancer? a dose–response meta-analysis of prospective studies. Archives of Gynecology and Obstetrics. 2017;295(2):467–479. doi: 10.1007/s00404-016-4263-y. [DOI] [PubMed] [Google Scholar]
- 11.Si C.-J., Shu L., Zheng P.-F., et al. Dietary patterns and endometrial cancer: A meta-analysis. European Journal of Cancer Prevention : The Official Journal of the European Cancer Prevention Organisation (ECP) 2017;26(4):336–345. doi: 10.1097/CEJ.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 12.Win A. K., Reece J. C., Ryan S. Family history and risk of endometrial cancer : A systematic review and meta-analysis. Obstetrics & Gynecology. 2015;125(1):89–98. doi: 10.1097/AOG.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 13.Haoula Z., Salman M., Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Human Reproduction. 2012;27(5):1327–1331. doi: 10.1093/humrep/des042. [DOI] [PubMed] [Google Scholar]
- 14.Tao X., Jiang A., Yin L., Li Y., Tao F., Hu H. Body mass index and age at natural menopause: a meta-analysis. Menopause (New York, NY) 2015;22(4):469–474. doi: 10.1097/GME.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q., Li Y. Y., Tu C., et al. Parity and endometrial cancer risk: a meta-analysis of epidemiological studies. Scientific Reports. 2015;5 doi: 10.1038/srep14243.14243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong T. T., Wang Y. L., Ma X. X. Age at menarche and endometrial cancer risk: a dose-response meta-analysis of prospective studies. Scientific Reports. 2015;5(1) doi: 10.1038/srep14051.14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen N., Peto R., Beral V., et al. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27 276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncology. 2015;16:1061–1070. doi: 10.1016/S1470-2045(15)00212-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhan B., Liu X., Li F., Zhang D. Breastfeeding and the incidence of endometrial cancer: a meta-analysis. Oncotarget. 2015;6(35):38398–38409. doi: 10.18632/oncotarget.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glisic M., Kastrati N., Musa J., et al. Phytoestrogen supplementation and body composition in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Maturitas. 2018;115:74–83. doi: 10.1016/j.maturitas.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Gold E. B. The timing of the age at which natural menopause occurs. Obstetrics & Gynecology Clinics of North America. 2011;38(3):425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaaks R., Lukanova A., Kurzer M. S. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiology, Biomarkers & Prevention : A Publication of The American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2002;11:1531–1543. [PubMed] [Google Scholar]
- 22.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. The Lancet Oncology. 2012;13(11):1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong G.-C., Liu Y., Chen N., et al. Reproductive factors, menopausal hormone therapies and primary liver cancer risk: a systematic review and dose-response meta-analysis of observational studies. Human Reproduction Update. 2016;23(1):126–138. doi: 10.1093/humupd/dmw037. [DOI] [PubMed] [Google Scholar]
- 24.Kanazir M., Boricic I., Delic D., et al. Risk factors for hepatocellular carcinoma: a case-control study in belgrade (serbia) Tumori. 2010;96(6):911–917. doi: 10.1177/548.6508. [DOI] [PubMed] [Google Scholar]
- 25.Mucci L. A., Kuper H. E., Tamimi R., Lagiou P., Spanos E., Trichopoulos D. Age at menarche and age at menopause in relation to hepatocellular carcinoma in women. British Journal of Obstetrics and Gynaecology. 2001;108(3):291–294. doi: 10.1016/s0306-5456(00)00032-2. [DOI] [PubMed] [Google Scholar]
- 26.Kvale G., Heuch I., Ursin G. Reproductive factors and risk of cancer of the uterine corpus: a prospective study. Cancer Research. 1988;48(21):6217–6221. [PubMed] [Google Scholar]
- 27.Shu X., Brinton L. A., Zheng W., Gao Y. T., Fan J., Fraumeni J. F. A population‐based case‐control study of endometrial cancer in shanghai, china. International Journal of Cancer. 1991;49(1):38–43. doi: 10.1002/ijc.2910490108. [DOI] [PubMed] [Google Scholar]
- 28.Brinton L. A., Berman M. L., Mortel R., et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. American Journal of Obstetrics & Gynecology. 1992;167(5):1317–1325. doi: 10.1016/S0002-9378(11)91709-8. [DOI] [PubMed] [Google Scholar]
- 29.Hirose K., Tajima K., Hamajima N., et al. Comparative case-referent study of risk factors among hormone-related female cancers in Japan. Japanese Journal of Cancer Research. 1999;90(3):255–261. doi: 10.1111/j.1349-7006.1999.tb00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson J. E., Sellers T. A., Anderson K. E., Folsom A. R. Does a family history of cancer increase the risk for postmenopausal endometrial carcinoma? a prospective cohort study and a nested case-control family study of older women. Cancer. 1999;85(11):2444–2449. doi: 10.1002/(SICI)1097-0142(19990601)85:11<2444::AID-CNCR20>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 31.Salazar-Martinez E., Lazcano-Ponce E. C., Lira-Lira G. G., Escudero-De Los Rios P., Salmeron-Castro J., Hernandez-Avila M. Reproductive factors of ovarian and endometrial cancer risk in a high fertility population in Mexico. Cancer Research. 1999;59(15):3658–3662. [PubMed] [Google Scholar]
- 32.Xu W.-H., Xiang Y.-B., Ruan Z.-X., et al. Menstrual and reproductive factors and endometrial cancer risk: results from a population-based case-control study in urban shanghai. International Journal of Cancer. 2004;108(4):613–619. doi: 10.1002/ijc.11598. [DOI] [PubMed] [Google Scholar]
- 33.Trentham-Dietz A., Nichols H. B., Hampton J. M., Newcomb P. A. Weight change and risk of endometrial cancer. International Journal of Epidemiology. 2006;35(1):151–158. doi: 10.1093/ije/dyi226. [DOI] [PubMed] [Google Scholar]
- 34.Wernli K. J., Ray R. M., Gao D. L., De Roos A. J., Checkoway H., Thomas D. B. Menstrual and reproductive factors in relation to risk of endometrial cancer in Chinese women. Cancer Causes & Control. 2006;17(7):949–955. doi: 10.1007/s10552-006-0034-6. [DOI] [PubMed] [Google Scholar]
- 35.Setiawan V. W., Pike M. C., Kolonel L. N., Nomura A. M., Goodman M. T., Henderson B. E. Racial/ethnic differences in endometrial cancer risk: the multiethnic cohort study. American Journal of Epidemiology. 2007;165(3):262–270. doi: 10.1093/aje/kwk010. [DOI] [PubMed] [Google Scholar]
- 36.Zucchetto A., Serraino D., Polesel J., et al. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. European Journal of Cancer Prevention. 2009;18(4):316–321. doi: 10.1097/CEJ.0b013e328329d830. [DOI] [PubMed] [Google Scholar]
- 37.Karageorgi S., Hankinson S. E., Kraft P., De Vivo I. Reproductive factors and postmenopausal hormone use in relation to endometrial cancer risk in the Nurses' Health Study cohort 1976-2004. International Journal of Cancer. 2010;126(1):208–216. doi: 10.1002/ijc.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amankwah E. K., Friedenreich C. M., Magliocco A. M., et al. Hormonal and reproductive risk factors for sporadic microsatellite stable and unstable endometrial tumors. Cancer Epidemiology, Biomarkers & Prevention. 2013;22(7):1325–1331. doi: 10.1158/1055-9965.EPI-13-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dashti S. G., Chau R., Ouakrim D. A., et al. Female hormonal factors and the risk of endometrial cancer in Lynch syndrome. Journal of the American Medical Association. 2015;314(1):61–71. doi: 10.1001/jama.2015.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung K. J., Park C., Yun Y. D., Jee S. H. Duration of ovarian hormone exposure and gynecological cancer risk in korean women: the korean heart study. Cancer Epidemiology. 2016;41:1–7. doi: 10.1016/j.canep.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Yang H. P., Murphy K. R., Pfeiffer R. M., et al. Lifetime number of ovulatory cycles and risks of ovarian and endometrial cancer among postmenopausal women. American Journal of Epidemiology. 2016;183(9):800–814. doi: 10.1093/aje/kwv308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sponholtz T. R., Palmer J. R., Rosenberg L., Hatch E. E., Adams-Campbell L. L., Wise L. A. Reproductive factors and incidence of endometrial cancer in U.S. black women. Cancer Causes & Control. 2017;28(6):579–588. doi: 10.1007/s10552-017-0880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International Journal of Surgery. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Wells G., Shea B., O'Connell D., et al. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. 2016, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 45.Higgins J. P. T., Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 46.Higgins J. P. T., Thompson S. G. Controlling the risk of spurious findings from meta-regression. Statistics in Medicine. 2004;23(11):1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 47.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal (Clinical research ed) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orsini N., Li R., Wolk A., Khudyakov P., Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. American Journal of Epidemiology. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harre F. E., Jr., Lee K. L., Pollock B. G. Regression models in clinical studies: determining relationships between predictors and response. Journal of the National Cancer Institute. 1988;80(15):1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 50.Orsini N., Bellocco R., Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal. 2006;6(1):40–57. doi: 10.1177/1536867X0600600103. [DOI] [Google Scholar]
- 51.Jackson D., White I. R., Thompson S. G. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta-analyses. Statistics in Medicine. 2010;29(12):1282–1297. doi: 10.1002/sim.3602. [DOI] [PubMed] [Google Scholar]
- 52.Wang J., Lv J., Wang W., Jiang X. Alcohol consumption and risk of periodontitis: a meta-analysis. Journal of Clinical Periodontology. 2016;43(7):572–583. doi: 10.1111/jcpe.12556. [DOI] [PubMed] [Google Scholar]
- 53.Palacios S., Henderson V. W., Siseles N., Tan D., Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric : The Journal of The International Menopause Society. 2010;13(5):419–428. doi: 10.3109/13697137.2010.507886. [DOI] [PubMed] [Google Scholar]
- 54.Parazzini F. Determinants of age at menopause in women attending menopause clinics in Italy. Maturitas. 2007;56(3):280–287. doi: 10.1016/j.maturitas.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Zhu D., Chung H.-F., Pandeya N., et al. Body mass index and age at natural menopause: an international pooled analysis of 11 prospective studies. European Journal of Epidemiology. 2018;33(8):699–710. doi: 10.1007/s10654-018-0367-y. [DOI] [PubMed] [Google Scholar]
- 56.Pettersson B., Adami H., Bergström R., Johansson E. D. Menstruation span--a time-limited risk factor for endometrial carcinoma. Acta Obstetricia et Gynecologica Scandinavica. 1986;65(3):247–255. doi: 10.3109/00016348609155179. [DOI] [PubMed] [Google Scholar]
- 57.Key T. J. A., Pike M. C. The dose-effect relationship between “unopposed” oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. British Journal of Cancer. 1988;57(2):205–212. doi: 10.1038/bjc.1988.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pike M. C., Pearce C. L., Wu A. H. Prevention of cancers of the breast, endometrium and ovary. Oncogene. 2004;23(38):6379–6391. doi: 10.1038/sj.onc.1207899. [DOI] [PubMed] [Google Scholar]
- 59.Akhmedkhanov A., Zeleniuch-Jacquotte A., Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer: review of the evidence and research perspectives. Annals of the New York Academy of Sciences. 2001;943:296–315. doi: 10.1111/j.1749-6632.2001.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 60.Elwood J. M., Cole P., Rothman K. J., Kaplan S. D. Epidemiology of endometrial cancer. Journal of the National Cancer Institute. 1977;59(4):1055–1060. doi: 10.1093/jnci/59.4.1055. [DOI] [PubMed] [Google Scholar]
- 61.Munafò M. R., Flint J. Meta-analysis of genetic association studies. Trends in Genetics : TIG. 2004;20(9):439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Quality assessment of included case-control studies. Table S2. Quality assessment of included cohort studies.