FIG 6.
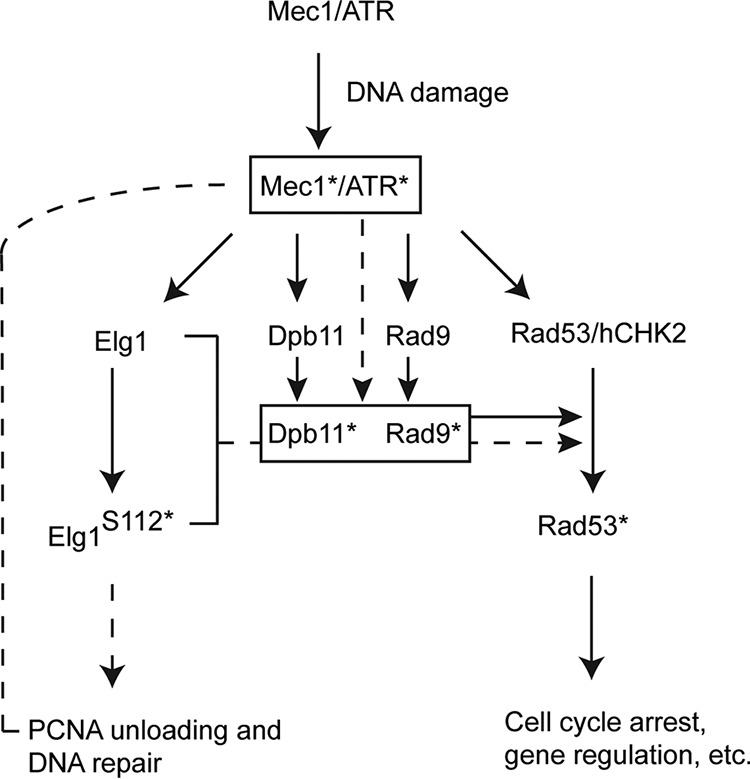
A model for the potential role of Elg1 in the activation of the DNA damage checkpoint. The current results are consistent with the following scenario: in response to DNA damage, Mec1 becomes activated (Mec1*). This, in turn, results in the phosphorylation of Rad53 (Rad53*) as well as serine 112 of Elg1 (Elg1 S112*). Phosphorylated Elg1 helps in the DNA repair process by unloading the chromatin-bound PCNA during DNA damage, whereas phosphorylated Rad53 transduces the signal to complete checkpoint functions related to cell cycle arrest. The checkpoint sensor (Dpb11) and mediator (Rad9) are phosphorylated (Dpb11* and Rad9*, respectively) by Mec1*, which in turn generates Rad53* through Dpb11*/Rad9*. The chromatin-bound PCNA unloading activity of Elg1 is necessary for Rad53 phosphorylation as absence of this step resulted in a failure to phosphorylate both Dpb11 and Rad9. The accumulated PCNA at the damaged site (here, the Lac operator site) probably controls the Dpb11*/Rad9* formation by blocking Mec1* activity (see details in Discussion) and hence controls the Rad53 kinase activity. Solid lines denote findings from previously published work, and dashed lines indicate the findings of the present study.
