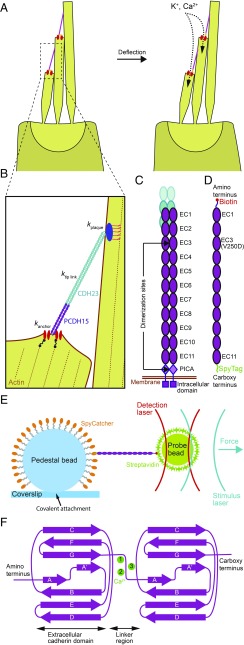
Fig. 1.
The role of tip link proteins in transduction by hair cells. (A) The hair bundle is a cluster of stiff, actin-filled protrusions called stereocilia that stands atop each hair cell in the inner ear. Each stereocilium is connected to its tallest adjacent neighbor through a proteinaceous filament called a tip link (pink), which is coupled at its base to mechanically gated ion channels (red). Deflection of a hair bundle increases the tension in the tip links, biasing the channels toward an open state that allows the influx of positively charged ions. (B) The mechanical element that converts hair bundle displacement into a force capable of opening the channels is called the gating spring. Its stiffness comprises the stiffnesses of the channel and its lower anchor (kanchor), the tip link proteins PCDH15 and CDH23 (ktip link), and the insertional plaque that anchors the link’s top end into the taller stereocilium (kplaque). (C) The mechanical properties of the tip link emerge from its quaternary structure and from the characteristics of its constituent proteins. The lower third of the link consists of a dimer of PCDH15 molecules, each of which includes 11 extracellular cadherin (EC) domains. (D) To measure the mechanical behavior of monomeric PCDH15, we tagged each end with a distinct molecular handle. We eliminated dimerization by a point mutation (V250D) in domain EC3 and by truncation of the PICA domain. (E) We probed the mechanics of a PCDH15 monomer by confining it through molecular handles between an immobile 2-μm glass pedestal bead and a diffusive 1-μm plastic probe bead. To acquire each force-extension relationship, we measured the position of the probe bead with a detection laser while applying a force with a stimulus laser. (F) The folding motifs of individual EC domains influence the mechanical properties of the full-length protein. Up to three calcium ions (green) can bind between successive domains.