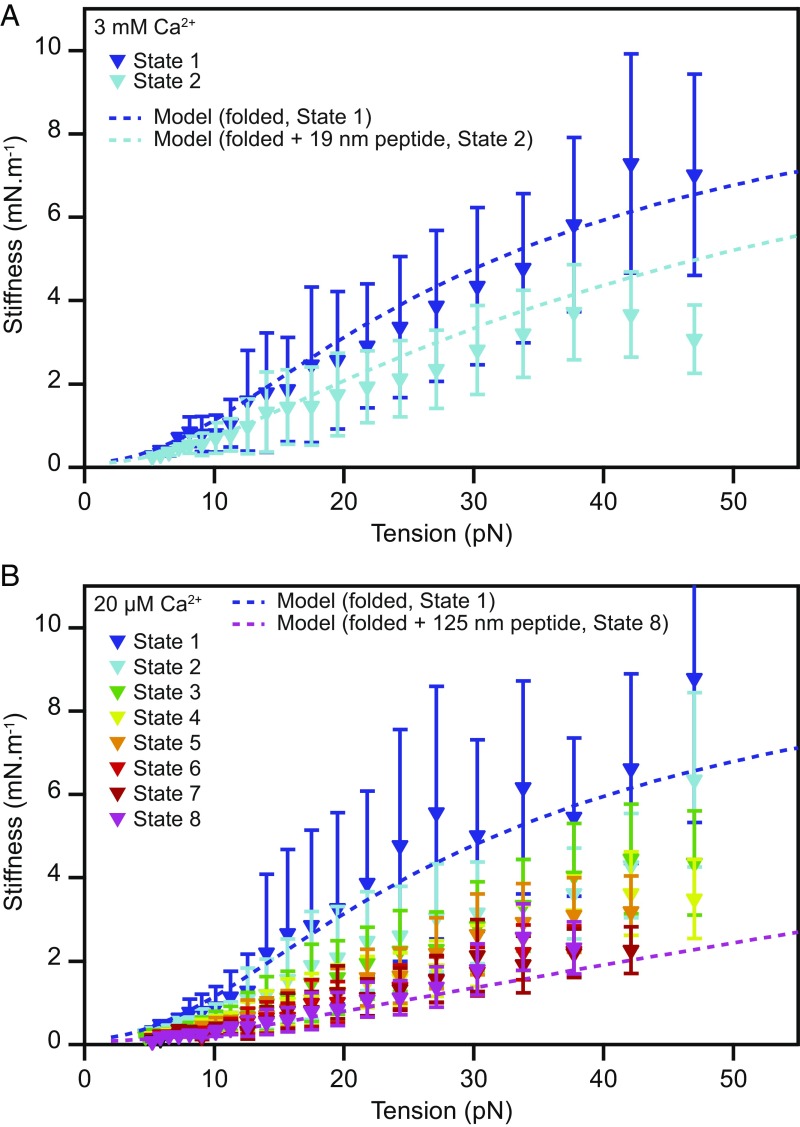
Fig. 3.
Stiffness of monomeric PCDH15. (A) The stiffnesses of the different conformational states of PCDH15 at a Ca2+ concentration of 3 mM correspond to the slopes of the highly occupied regions of the state space in Fig. 2 D and E and are corrected for the stiffness of the molecular tags and anchors. The dark-blue dashed line represents the stiffness of our model of state 1, the fully folded protein, with the parameter values of Table 1 (b = 3.0 nm; lclinker = 1.35 nm; kfolded = 10 mN·m−1). Parameter values were averaged over both Ca2+ concentrations. The light-blue dashed line represents the model for state 2, with an additional 19-nm segment of unfolded protein with a persistence length of 0.49 nm representing the combined effect of the events AU and BU. (B) The corresponding data for a Ca2+ concentration of 20 μM capture a variety of unfolding events leading to states 2–8. The dark-blue dashed line represents a model of the fully folded protein (state 1); the pink dashed line depicts the modeled stiffness of the protein in state 8, with an unstructured 125-nm-long peptide to represent the unfolding of three cadherin domains in series with contour length changes of 15 nm and 4 nm. The experimental data are mean ± SEM for five molecules at a Ca2+ concentration of 3 mM and for six molecules at a Ca2+ concentration of 20 µM.