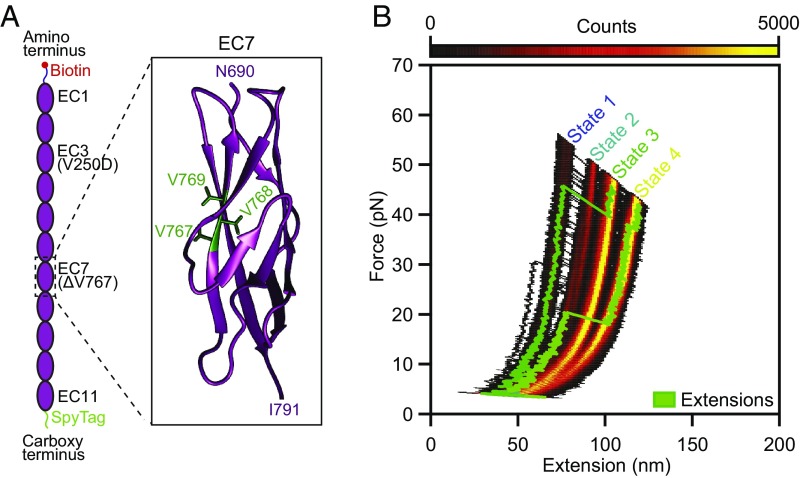
Fig. 5.
Effect of a hearing loss-associated mutation on PCDH15 mechanics. (A) We deleted V767 in the seventh EC domain of PCDH15. As indicated in the crystal structure (Protein Data Bank ID code 5W1D; image generated with UCSF Chimera), V767 is located in the F strand of the cadherin fold. (B) A state-space heatmap for 500 extension-relaxation cycles reveals that at a Ca2+ concentration of 3 mM the mutant protein can assume four distinct conformational states. The two additional states not observed in the wild-type protein result from unfolding of the pathological cadherin domain in series with the usual states 1 and 2. Unfolding of the pathological domain is rare and occurs in only a few cycles, two of which are superimposed on the heat map (green traces). The waiting time between cycles was 0.2 s.