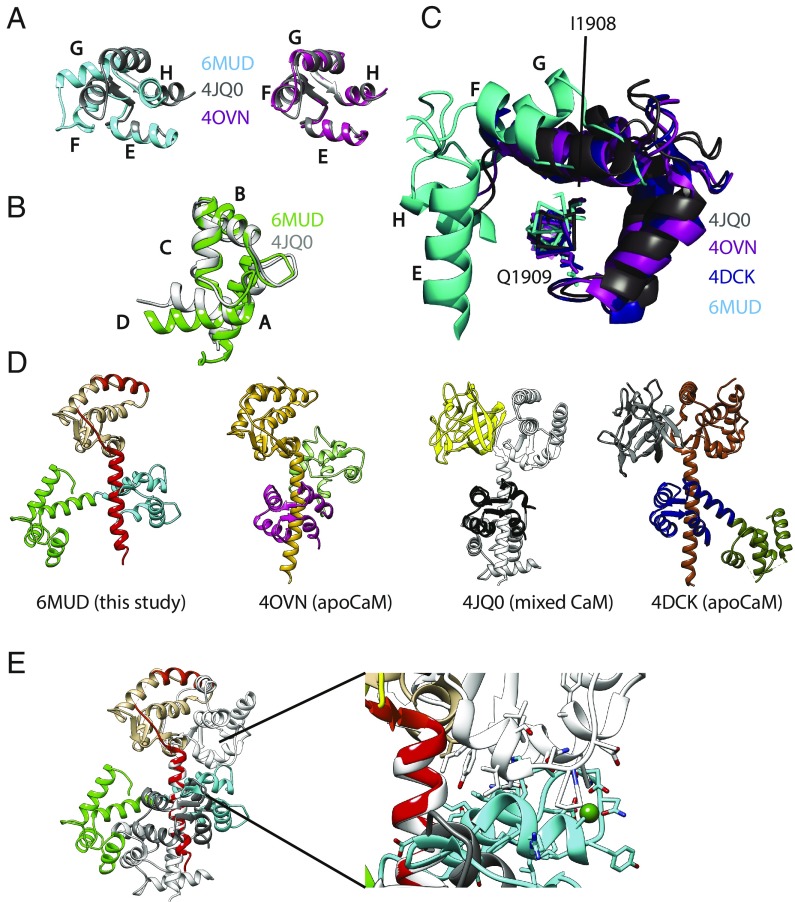
Fig. 2.
Structural comparison. (A) Comparisons of the C-lobe conformation from this study (PDB ID code 6MUD, blue), a C-lobe from a previously proposed NaVCT:Ca2+/CaM complex (PDB ID code 4JQ0, gray), and an apoC-lobe (PDB ID code 4OVN, magenta), which shows that the previously proposed Ca2+/C-lobe from a NaV CT complex (13) is an apoC-lobe, not a Ca2+/C-lobe. The letters in A–C correspond to the CaM helices. (B) Comparisons of the N-lobe from this study (green) with the N-lobe from PDB ID code 4JQ0 (gray), confirming that the latter represents a Ca2+-occupied N-lobe. (C) Superpositions showing the relative C-lobe positions found in this study (6MUD, cyan) and three other previous CaM complex with the NaV1.5 CT. (D) Side-by-side comparison of the current structure (PDB ID code 6MUD) and three other CaM complexes, including two apo-CaM complexes (PDB ID codes 4OVN and 4DCK) and one previously proposed Ca2+/CaM complex, which is a mixed CaM (PDB ID code 4JQ0). The structures are shown in the exact same view, based on a superposition of the sixth helix immediately after the EF-hand domain. (E) Superposition of the Ca2+/CaM (PDB ID code 6MUD) and mixed CaM (PDB ID code 4JQ0) complexes with NaV1.5CT, based on the sixth helix containing the IQ domain, using the same colors as in D. This shows that the Ca2+/C-lobe (cyan) from the NaV1.5 CT: Ca2+/CaM structure would clash with the NaV1.5 EF-hand domain in the mixed CaM complex (PDB ID code 4JQ0). Therefore, binding of the Ca2+/C-lobe to the IQ domain is prevented by the FHF, which utilizes reorientation of the EF-hand domain as a mechanism to prevent Ca2+/C-lobe binding.