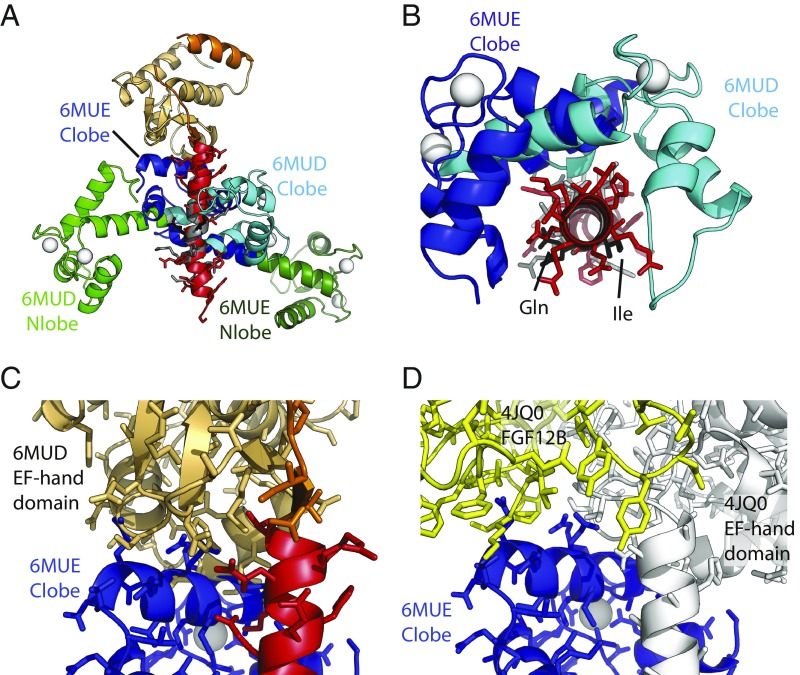
Fig. 7.
Differences in IQ motif binding between Nav1.5 and Nav1.4. The structures were superposed based on NaV CT helix 6, containing the IQ domain. (A) Superposition of the complexes NaV1.5 CT:Ca2+/CaM (PDB ID code 6MUD, this study) and NaV1.4 IQ domain:Ca2+/CaM (PDB ID code 6MUE, this study). N-lobe and C-lobe are shown in green and blue, respectively, with light colors for the NaV1.5 complex and dark colors for the NaV1.4 complex. NaV1.5 EF-hand domain (beige), NaV1.5 IQ domain (red), and NaV1.4 IQ domain (gray) are shown. Calcium ions are shown as white spheres. (B) Same superposition as in A but with the view from the N terminus of the IQ domain toward the C terminus. The EF-hand domain has been omitted and only the C-lobes are shown. This indicates a ∼90° rotation of the C-lobe binding to NaV1.4 compared with the NaV1.5 IQ domain. The Ile and Gln residues of the “IQ” motif are shown in black for reference. (C) Same superposition as in A, but only showing the NaV1.5 CT (EF-hand domain in beige; IQ domain in red) and the C-lobe from the NaV1.4 complex. This shows that the C-lobe would clash with the EF-hand domain, suggesting that, for it to bind in this mode, the EF-hand domain in NaV1.4 would have to be displaced. (D) Superposition, based on helix 6, for the NaV1.5 CT: mixed-CaM complex with FGF12B (PDB ID code 4JQ0) and for the NaV1.4 IQ: Ca2+/CaM complex (PDB ID code 6MUE, this study). Shown are the NaV1.5 CT (white) and FGF12B (yellow) from 4JQ0, and the C-lobe from the NaV1.4 complex. This shows that, also in the presence of an FHF, there would be clashes between the Ca2+/C-lobe and the EF-hand domain. In addition, clashes would occur between Ca2+/Clobe and FGF12B.