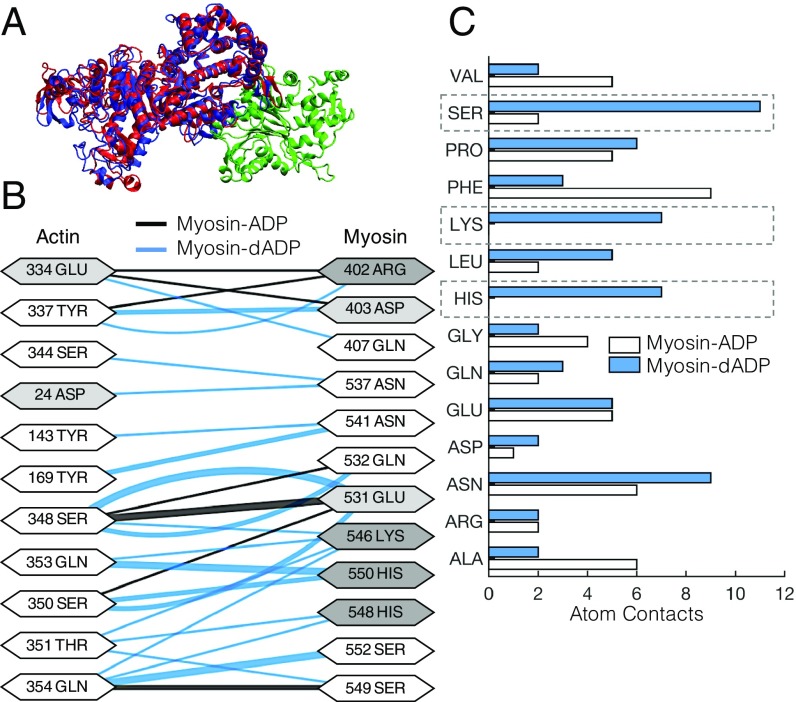
Fig. 1.
dADP acts as an allosteric myosin effector to promote polar interactions between actin and prepowerstroke myosin. (A) Actin monomer (green) and either ADP-bound (red) or dADP-bound (blue) myosin S1 segments. (B) Contact pairs of residues between actin and ADP-bound myosin (black lines) and dADP-bound myosin (blue lines) for an actin–myosin complex. The thickness of the line connecting the interacting residues corresponds to the number of atoms in contact between the residues, and the shading of the residue indicates whether it is polar (white), acidic (light gray), or basic (dark gray). (C) Number of atoms in “contact” (discussed in the text) between actin and myosin-ADP (white bars) and myosin-dADP (blue bars) in a prepowerstroke actin–myosin protein complex. There are significant increases in atom contacts that consist of polar residues (SER, LYS, and HIS; highlighted by dashed boxes) between actin and myosin-dADP compared with actin and myosin-ADP.