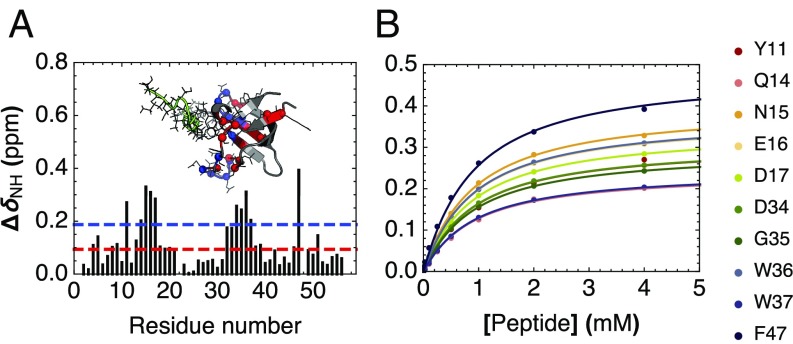
Fig. 4.
Peptide binding of cSH3 by NMR spectroscopy. (A) Chemical shift perturbations (CSPs) of backbone 1H–15N HSQC cross-peaks resulting from addition of a 20-fold excess unlabeled peptide to 15N-labeled consensus SH3. The red and blue lines indicate 1 and 2 SDs, respectively. Inset shows a homology model of cSH3 aligned to a peptide-bound structure of human Fyn SH3 (PDB 1A0N; peptide shown in green). Residues showing CSPs greater than 1 and 2 SDs are shown with α-carbons as spheres and side-chain atoms as lines, and colored red and blue, respectively. (B) Binding isotherms for the 10 consensus SH3 residues showing largest CSPs. The solid lines are obtained from a global fit to a single-site binding model using a common dissociation constant for all residues.