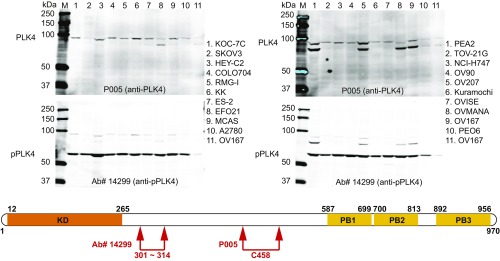
Fig. 3.
Western immunoblot of 19 different ovarian cell lines and one colorectal (NCI-H747) cancer cell line demonstrates full-length PLK4 (Upper Left and Upper Right) and phospho-PLK4 (Lower Left and Lower Right). (Upper) Western blots demonstrate full-length (97-kDa) PLK4 with a commercially available anti-PLK4 antibody (P005; Cell Signaling 3258, recognizing an epitope centered on the cysteine458 amino acid). (Lower) Western blots were performed by using the same filter after washing with buffer and reprobing with an anti–phospho-serine305-PLK4 antibody (Ab#14299). Please note that the phospho-PLK4 in the lower filter is of substantially smaller size, ∼60 kDa. The difference is appreciated as the full-length signals were not completely removed by stripping in the lower filters. Cultured cells were not synchronized with regard to cell cycle. We confirmed that the 60-kDa band corresponded to pPLK4 by removing this band from the gel and performing amino acid sequence analyses. We also confirmed that a second anti–phospho-PLK4 antibody (Ab#3) from our laboratory recognized phospho-PLK4 by using immunoprecipitation assays followed by PAGE with silver staining to identify the band recognized on Western blot and, finally, MS to assess the amino-acid sequence of protein band (amino-acid sequence provided in Results). SI Appendix, Fig. S1 shows results in 23 additional colorectal and 9 breast cancer cell lines. (Lower) Schematic illustration of PLK4 amino-acid sequence shows the relative locations of epitopes recognized by the two antibodies used for Western immunoblot analyses (Upper), one from the laboratory of G.P.N. recognizing phospho-serine305-PLK4, produced in collaboration with Cell Signaling Technology, and the second commercially available recognizing cysteine458-PLK4.