Figure 2.
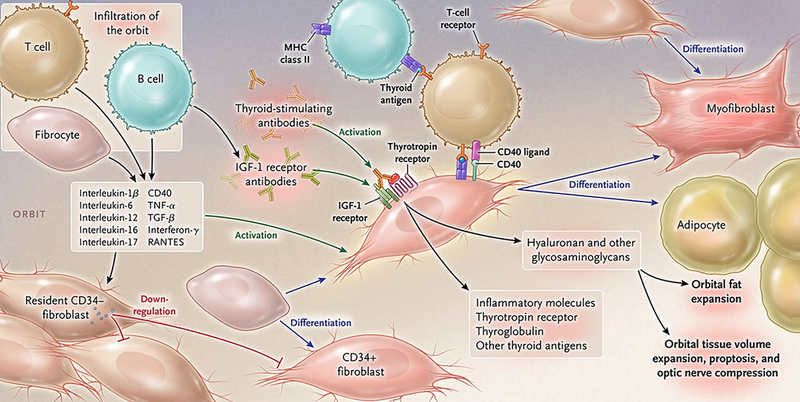
Theoretical representation: the pathogenesis of thyroid-associated ophthalmopathy. The orbit becomes infiltrated by B and T cells and CD34+ fibrocytes uniquely in thyroid-associated ophthalmopathy. Bone marrow-derived fibrocytes express several proteins traditionally considered “thyroid-specific”. They can differentiate into CD34+ fibroblasts which in turn can further develop into myofibroblasts or adipocytes depending upon the molecular cues they receive from the tissue microenvironment. CD34+ fibroblasts cohabit the orbit with residential CD34- fibroblasts. These heterogeneous populations of orbital fibroblasts can produce cytokines under basal and activated states. These include interleukins 1β, 6, 8, 10, 16, IL-1 receptor antagonists, tumor necrosis factor α, the chemokine known as “regulated on activation, normal T expressed and secreted” or RANTES, CD40 ligand and several other cytokines and chemokines. These cytokines can act on infiltrating and residential cells. Like fibrocytes, CD34+ fibroblasts express thyrotropin receptor, thyroglobulin, and other thyroid proteins but at substantially lower levels. Thyroid-stimulating immunoglobulins and potentially other autoantibodies directed specifically at the insulin-like growth factor-I receptor, activate the thyrotropin/insulin-like growth factor receptor-1 complex, resulting in the activation of several downstream signaling pathways and expression of target genes. Orbital fibroblasts synthesize hyaluronan leading to increased orbital tissue volume. This expanded tissue can result in proptosis and optic nerve compression. Orbital fat also expands from de novo adipogenesis. From N. Engl. J. Med, Smith T.J. and Hegedus L., Graves’ Disease, 375; 1552–1565. Copyright (2016) Massachusetts Medical Society. Reprinted with permission.
