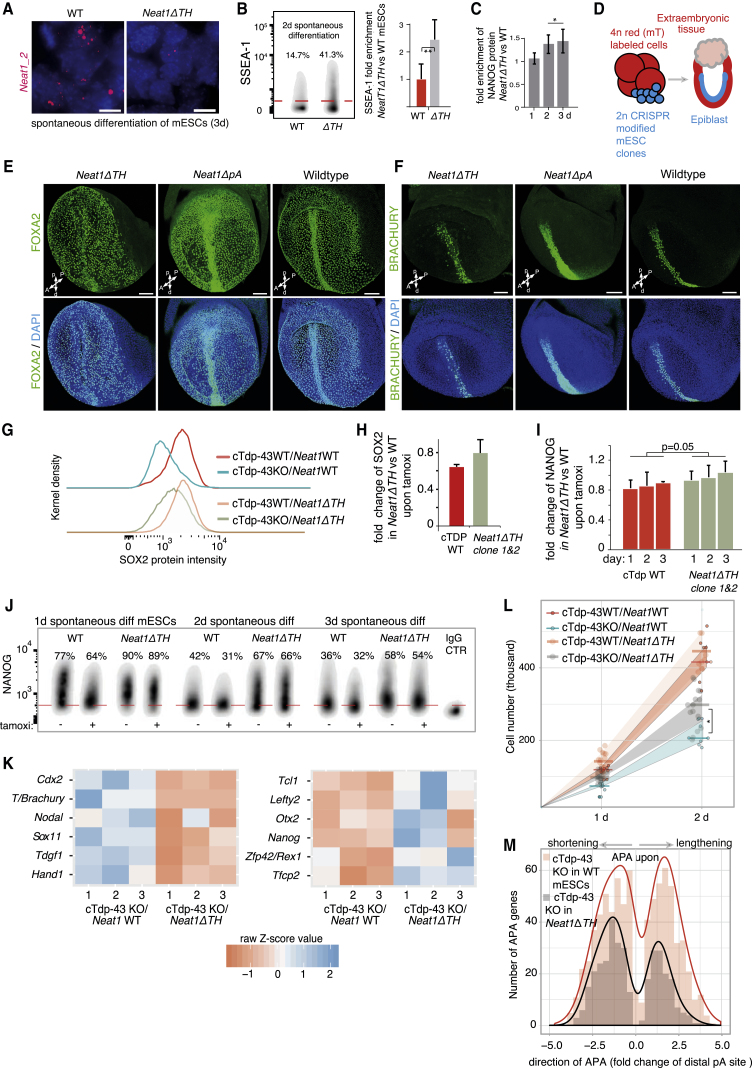
Figure 6.
Efficient Dissolution of Pluripotency upon Depletion of TDP-43 Requires Paraspeckles
(A) Representative photomicrographs demonstrating the downregulation of Neat1_2 paraspeckles in spontaneously differentiating mESCs by deletion of the triple helix (ΔTH) in the 3′ region (further results in Figure S6A). Red, Neat1_1 and_2 probes; blue, DAPI (nuclear stain). Scale bars, 10 μm.
(B and C) Pluripotency assessment by SSEA-1 (B, right, quantified gated positive cells) and intracellular NANOG (C) flow cytometry of spontaneously differentiating Neat1ΔTH and WT mESCs (duration indicated). IgG-treated samples were used for gating positive cells (red line in B). Error bars, SD; two-sided t test; biological replicates, n = 3 per time point; ∗p < 0.05, ∗∗p < 0.01.
(D–F) In vivo analysis of the developmental potency of mESCs exhibiting downregulation of paraspeckles using a 2n mESC - 4n aggregated mouse embryo complementation assay, giving rise, respectively and exclusively, to embryonic and extraembryonic tissues (D). Shown are mouse embryos (E7.75–E8.0) resulting from aggregations of Neat1ΔTH and Neat1ΔpA mESCs with 4n 2- to 4-cell-stage embryos and representative analysis of FOXA2 (E) and BRACHYURY (F) by immunostaining (n = 7 for Neat1ΔTH [5 are shown in Figures S6B and S6C], n = 7 for Neat1ΔpA [2 are shown in Figure S6D], and n = 3 for parental control mESC [2 are shown in Figure S6E]). Non-manipulated embryos are shown on the right. Blue, DAPI (nuclear stain). Scale bars, 100 μm. A, anterior; P, posterior; p, proximal; d, distal.
(G–L) Pluripotency assessment by intracellular immunostaining flow cytometry (G–J; error bars, SD, two-sided t test in I, n = 3), RNA-seq (K; n = 3/group, Fisher’s exact test), and growth kinetics (L; Mann-Whitney U test, n ≥ 8/group as indicated by dots) in differentiating parental cTdp-43 KO mESCs or the same line harboring Neat1ΔTH, treated with tamoxifen during 2.5 days of spontaneous differentiation or left untreated (all consisting of independent replicates). Also shown are representative flow cytometry plots of SOX2 (G) and quantification of gated positive cells according to the IgG control (H) and of NANOG (I and J). IgG-treated samples were used to gate the positive cells (dotted red line) and to quantify the enrichment of these cells. In (K), shown are up- and downregulated representative differentiation and pluripotency genes according to the ScoreCard panel (Tsankov et al., 2015, and Kalkan et al., 2017, respectively), comparing the impact of Tdp-43 KO in mESCs lacking Neat1_2 (Neat1ΔTH) with control mESCs harboring WT Neat1. In (L), the width of colored intervals represents the interquartile range of the growth kinetics measurements.
(M) Histogram depicting rearrangements (direction and degree) of pA sites following cTdp-43 KO in Neat1ΔTH mESCs compared with the respective parental WT cells (genes passing filtering and statistical analysis as outlined in Figures S3C–S3E; APA in the range of ±5-fold change, p < 0.01). mESCs were treated with tamoxifen for 2.5 days or left untreated during spontaneous differentiation (n = 3 independent replicates/group).