Table 1.
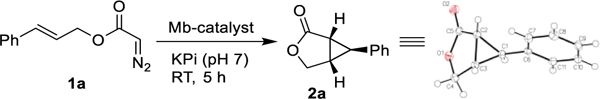
Intramolecular cyclopropanation of cinnamyl 2-diazoacetate (1a) with Mb and variants thereof.a
 | |||||
|---|---|---|---|---|---|
| Entry | catalyst | OD600 | Yieldb | TON | e.e. |
| 1 | Mb | - | 13% | 32 | 80% |
| 2 | Mb(L29A) | - | 65% | 163 | 93% |
| 3 | Mb(V68A) | - | 65% | 162 | 90% |
| 4 | Mb(H64V,V68A) | - | 33% | 82 | 81% |
| 5 | Mb(L29A,H64V,V68A) | - | 99% | 250 | 96% |
| 6c | Mb(L29A,H64V,V68A) | 40 | 74% | 185 | 96% |
| 7d | Mb(L29A,H64V,V68A) | 40 | 99% | 90 | 96% |
| 8 | Mb(H64V,I107S) | - | 78% | 195 | 97% |
| 9d | Mb(H64V,I107S) | 40 | 99% (83%)e |
90 | >99% |
Reaction conditions: 5 mM cinnamyl 2-diazoacetate (1a), 20 μM Mb variant (or C41(DE3) E. coli cells at indicated OD600) in KPi buffer (50 mM, pH 7), 10 mM Na2S2O4 (protein only), r.t., 5 hours in anaerobic chamber.
GC yield.
15 min reaction time.
Using 2.5 mM 1a.
Isolated yield.
