Scheme 9.
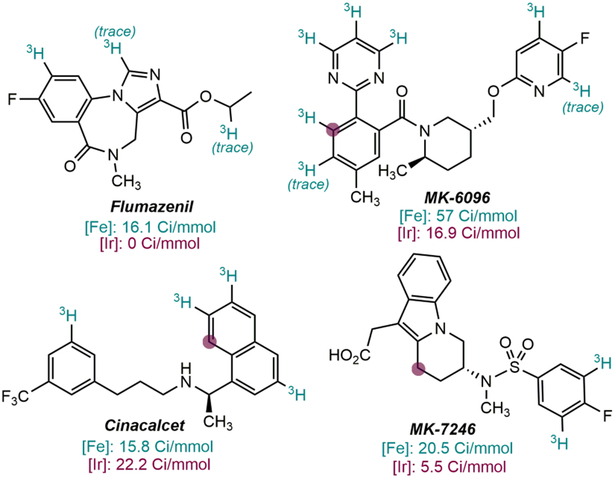
Drugs tritiated with (H4-iPrCNC)Fe(N2)2 (reference 61). Positions labelled with [Ir(COD)(pyridine)(PCy3)]PF6 are highlighted with a purple dot. Specific activities are reported in green for Fe and red for Ir below each drug. Conditions for (H4-iPrCNC)Fe(N2)2: 25 mol% catalyst loading, 7 μmol substrate, 1.2 Ci 3H2 (0.15 atm), 0.2 ml NMP, 23 °C, 16 h. Conditions for [Ir(COD)(pyridine)(PCy3)]PF6: 25 mol% catalyst loading, 7 μmol substrate, 1.2 Ci 3H2 (0.15 atm), 0.5 ml CH2Cl2, 23 °C, 16 h. The sodium salt conjugate base was used for the tritiation of MK-7246 owing to incompatibility of the carboxylic acid functionality with (H4-iPrCNC)Fe(N2)2.