Fig. 5.
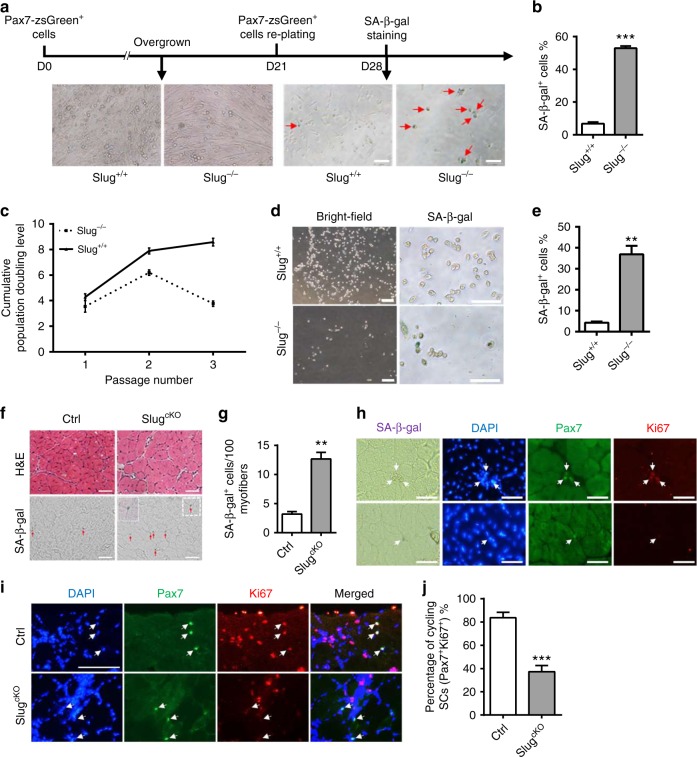
Slug-deficient SCs acquire senescence properties during self-renewal. a SA-β-Gal staining. SCs of indicated genotype were differentiated for 21 days. 5000 zsGreen+ reserve cells were sub-cultured for another 7 days and subject to SA-β-Gal staining. Red arrows indicate SA-β-Gal+ cells. Scale bar, 100 µm. b Quantification of the percentage of SA-β-Gal+ cells stained in a. ***p < 0.001 by student’s t-test. c Cumulative population doubling level (CPDL) obtained in cultures of primary SC-derived myoblasts from Slug+/+ and Slug−/− mice. d Photographs (left) and SA-β-Gal staining (right) of SC-derived myoblasts on day 7 of culture at passage 3. Scale bar, 200 µm (left); 100 µm (right). e Percentage of the SA-β-Gal+ cells in d. **p < 0.01 by student’s t-test. f Representative images of H&E (upper) and SA-β-Gal staining (lower) on transverse TA muscle cryosections. TA muscles from SlugcKO and Ctrl mice (n = 3 per genotype) were harvested on day 10 post injury and subject to H&E and SA-β-Gal staining. Red arrows indicated SA-β-Gal+ cells. The window (lower panel, right picture) represents high magnification of dotted boxed area. Scale bar, 50 µm. g Quantification of the SA-β-Gal+ cell numbers stained in f. **p < 0.01 by student’s t-test. h Senescent SCs in injured mice of SlugcKO and Ctrl mice. SA-β-Gal staining was combined with IHC staining against Pax7 and Ki67 on TA muscle cryosections being treated as described in f. Scale bar, 50 µm. i IHC staining for Pax7 and Ki67 in TA muscle sections harvested at day 2.5 post the second BaCl2 injury (n = 3 mice per group). Arrows indicated SCs in the section. Scale bar, 100 μm. j Percentage of cycling (Pax7+Ki67+) SCs stained in i. ***p < 0.001 by student’s t-test. Data are presented as mean ± SEM of three independent experiments. Also see Supplementary Fig. 12. Source data are provided as a Source Data file