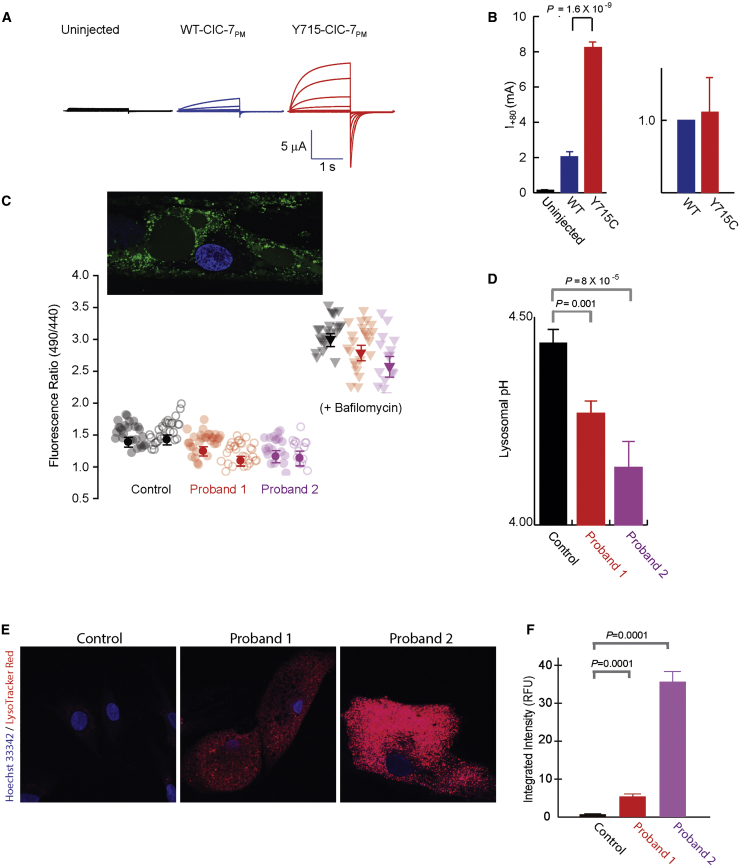
Figure 2.
Cellular pH and Chloride Transport Studies
(A) ClC-7 p.Tyr715Cys expression causes increased current in Xenopus oocytes. Two-electrode voltage-clamp current traces are shown for uninjected (black), WT ClC-7PM (blue), and p.Tyr715Cys ClC-7PM (red) in response to a series of 1 s voltage steps from −100 mV to + 80 mV in increments of 20 mV, followed by a step to −80 mV for 1.4 s before return to a holding potential of −30 mV.
(B) Bar graphs of current (+/− SEM, measured at +80 mV) or surface expression of uninjected (black), WT ClC-7PM, or p.Tyr715Cys ClC-7PM. For current measurements, n = 3 (uninjected), 8 (WT), or 6 (p.Tyr715Cys) from a single batch of oocytes; the experiment was reproduced 11 times in separate oocyte batches with essentially identical results. For surface expression, five separate surface biotinylation experiments were performed (with 20 each of WT and p.Tyr715Cys oocytes); intensities were normalized by the WT band intensity and averaged. The currents shown in (A) were from one of the same batches of oocytes used for the surface-expression experiments.
(C) Lysosomal pH in control and proband fibroblasts was measured with OG488 ratiometric imaging. Raw fluorescence ratios are shown for individual cells (pale circles) and averages (+/− SEM) of n = ∼50 cells (dark circles) from two independent experiments each on control neonatal fibroblasts (1.54 ± 0.1) or primary fibroblasts from either proband (proband 1, 1.30 ± 0.07; proband 2, 1.28 ± 0.08). Ratios from bafilomycin-treated cells from each are also shown as a negative control. The lower the 490/440nm fluorescence ratio, the lower the pH. An image of an OG488-treated proband cell is shown in the inset.
(D) Averaged lysosomal pH obtained from the experiments shown in part (C) (control, 4.44 ± 0.03; proband 1, 4.26 ± 0.03; proband 2, 4.19 ± 0.06). Separate calibrations were performed for each experiment and used for converting the ratios to pH values (Figure S3). Error bars indicate SEM; p values as shown.
(E) Cultured fibroblasts incubated with LysoTracker Red DND-99. Fluorescent intensity was significantly greater in cells of probands 1 and 2 than in control cells. Nuclei are stained with Hoechst (blue channel).
(F) Mean (+/− SEM) integrated intensity of LysoTracker Red DND-99 (red channel) per cell is plotted in relative fluorescence units (RFU); values are 107. Nuclei are stained with Hoechst (blue channel). Fluorescent intensity was significantly greater in cells of probands 1 and 2 than in control cells.
Abbreviations are as follows: SEM, standard error the mean; WT, wild-type.