Fig. 3.
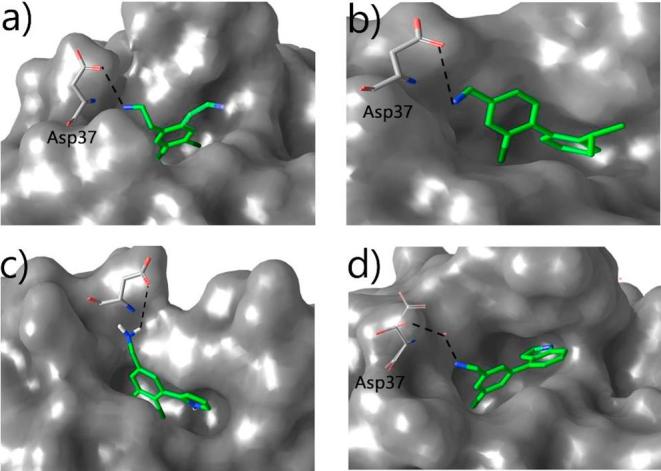
A side-by-side depiction of five fragments binding in the interface site of CK2α. a) NMR154L binds so that the dichloro moiety anchors the fragment into the hydrophobic pocket (pdb: 5CLP). The ethylamino group juts out of the pocket to form an H-bond with Asp37 outside the pocket. b) Compound 3 maintains the H-bond with Asp37. The second aromatic ring provides a better hydrophobic interaction in the pocket as it occupies more of the space. c) Compound 6 shows some promise as an interface binder. The interaction with Asp37 is maintained as well as the biaryl core occupying the width of the pocket. It is clear from the image there is an unoccupied area of the pocket beneath the pyrrole ring. This is a potential area for growth. d) The lead fragment CAM187 (7), like its predecessors, forms an H-bond with Asp37. This time with the aid of a bridging water molecule. In indole moiety provides the best hydrophobic interactions out of the molecules tested. It provides both the with to fill the pocket as well as the depth to fill it.
