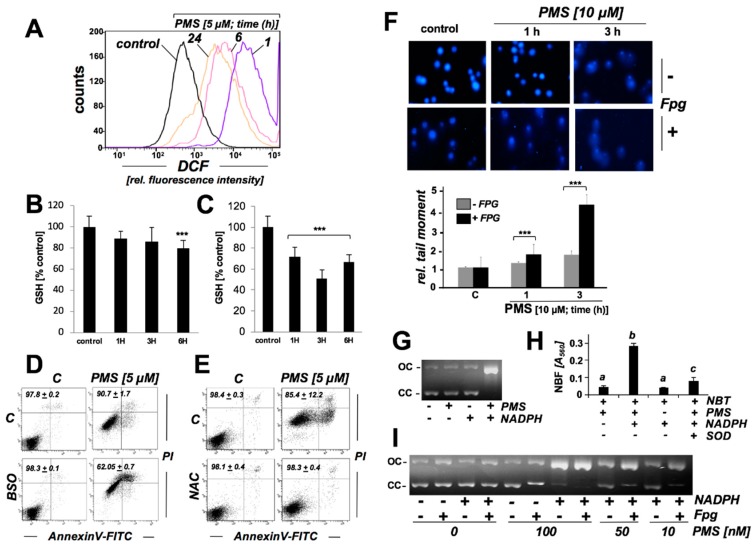
Figure 5.
PMS exposure induces redox dysregulation with glutathione depletion and oxidative impairment of genomic integrity in human A375 malignant melanoma cells, a genotoxic effect that can also be observed in an in vitro plasmid cleavage assay. (A) Analysis of oxidative stress in PMS-exposed A375 cells (10 µM, ≤ 24 h) as monitored by flow cytometric detection of DCF fluorescence. One set of histograms representative of three repeats is shown. (B,C) Time course (≤ 6 h) of PMS-modulation of intracellular reduced glutathione content in A375 cells (panel B: 1 µM; panel C: 10 µM), normalized to cell number (mean ± SD, n ≥ 3; *** p < 0.001). (D,E) After preincubation (24 h) with BSO (1 mM, panel C) or NAC (10 mM, panel D), PMS-induced (5 µM, 24 h) A375 cell death was assessed by flow cytometric analysis of AV/PI-stained cells (mean ± SD, n ≥ 3). (F) Fpg-enhanced Comet assay assessing cellular genotoxic stress (40 fold magnification; PMS: 10 µM; (analysis post treatment: ≤ 3 h); bar graph: tail moment analysis (n ≥ 100 per group; mean ± S.E.M; *** p < 0.001). (G) PMS-induced ΦX174-plasmid cleavage in vitro (OC: open circular; CC closed circular; PMS: 1 µM; NADPH: 200 µM; 3 h reaction time). (H) PMS-induced superoxide production as assessed by NBT reduction performed in the presence or absence of SOD (3000 μ/mL; n = 3). NBF was detected photometrically (A560 nm). (I) PMS dose response of Fpg-enhancement of ΦX174-plasmid cleavage in vitro (OC: open circular; CC closed circular; PMS: ≤ 100 nM; NADPH: 200 µM; 3 h reaction time), indicative of oxidative DNA lesions induced by NADPH/PMS exposure.