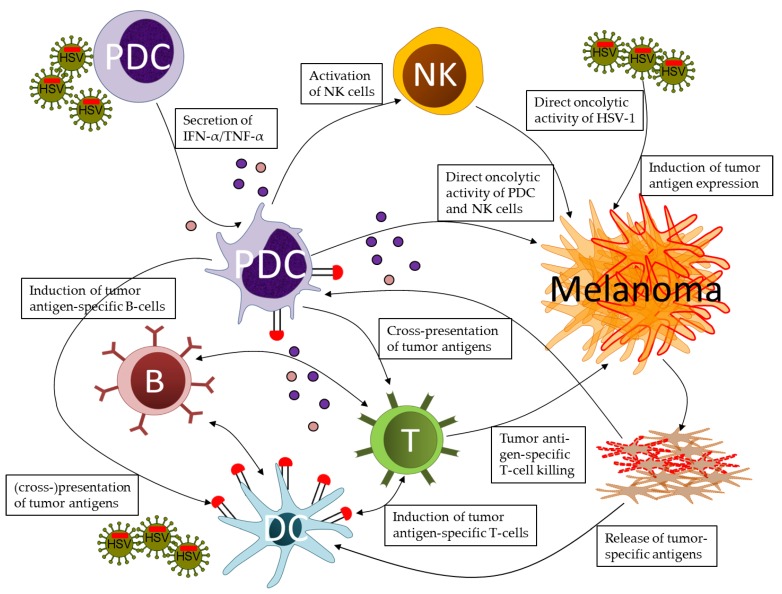
Figure 1.
Prospects of plasmacytoid dendritic cells (pDC) in orchestrating innate and adaptive immune responses against melanoma in the context of oncolytic herpes simplex virus (HSV) infections. Upon stimulation by wild type, attenuated, or replication-deficient HSV-1, pDC secrete IFN-alpha and TNF-alpha, which activate natural killer (NK) cells. NK cells and activated pDC attack the tumor cells via soluble and cell-associated cytotoxic mechanisms. These effects contribute to melanoma cell death induced by infection with oncolytic HSV-1, either via lytic replication or induction of apoptotic and necrotic cell death. Dying tumor cells release melanoma-associated antigens, which are cross-presented to T-cells by classical dendritic cells (DC), mostly cDC1, and, at least in part, by pDC. The close cooperation of pDC and DC results in the induction of tumor antigen-specific CD4+ and CD8+ T-cells, which contribute to long-term control of tumor cells. Tumor escape from immune responses may result in loss of tumor antigen expression, which may be counteracted by oncolytic HSV encoding for these antigens. Oncolytic HSV-1 expressing either self-antigens or neo-antigens may further serve as tumor vaccines in adjuvant and neo-adjuvant applications.