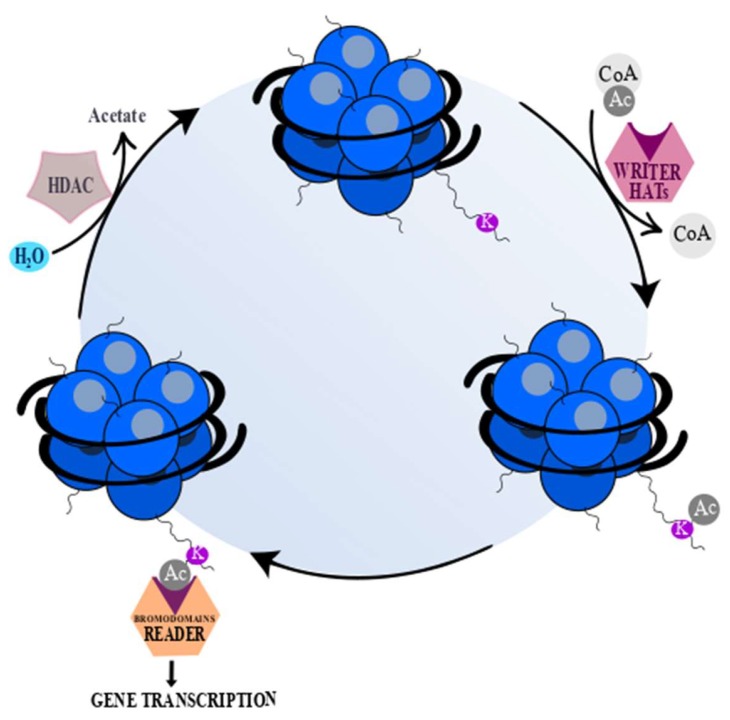
Figure 2.
“Writers”, “readers”, and “erasers”. “Writers” (histone acetyltransferases (HATs)) (pink) catalyze the transfer of an acetyl group to a histone lysine (K) or arginine residue. “Erasers” (histone deacetylase proteins (HDACs)) (gray) catalyze removal of an acetyl group from acetylated lysine or arginine. “Readers” (orange) possess specialized domains which are able to recognize and interact with certain histone modifications [31,32]. The acetyl (Ac) groups are added by HATs and removed by HDACs. Acetyl coenzyme A (Acetyl-CoA) serves as a donor of Ac groups and it is mainly obtained through ATP-citrate lyase reaction, under which mitochondrial-derived citrate is converted to acetyl-CoA and oxaloacetate. The changes of ATP-citrate lyase activity lead to variable acetyl-CoA accessibility and as a consequence global histone acetylation [20]. The deacetylation by HDACs yields deacetylated peptide and free acetate [20,33]. However, sirtuins (Class III HDACs), except for deacetylated peptide, generate a mixture of 2′- and 3′-O-acetyl-ADP ribose and nicotinamide [33].