Figure 2.
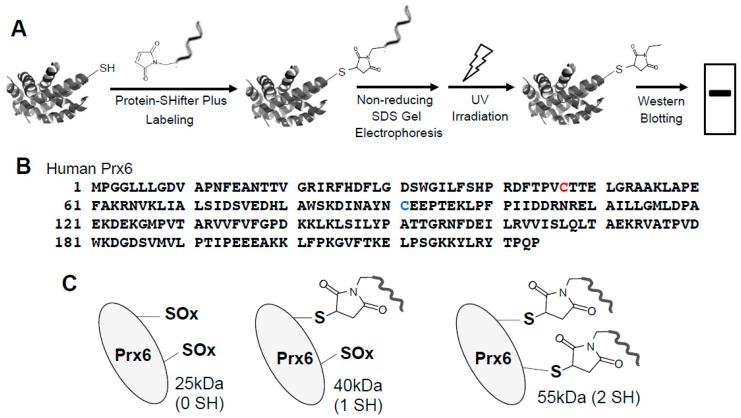
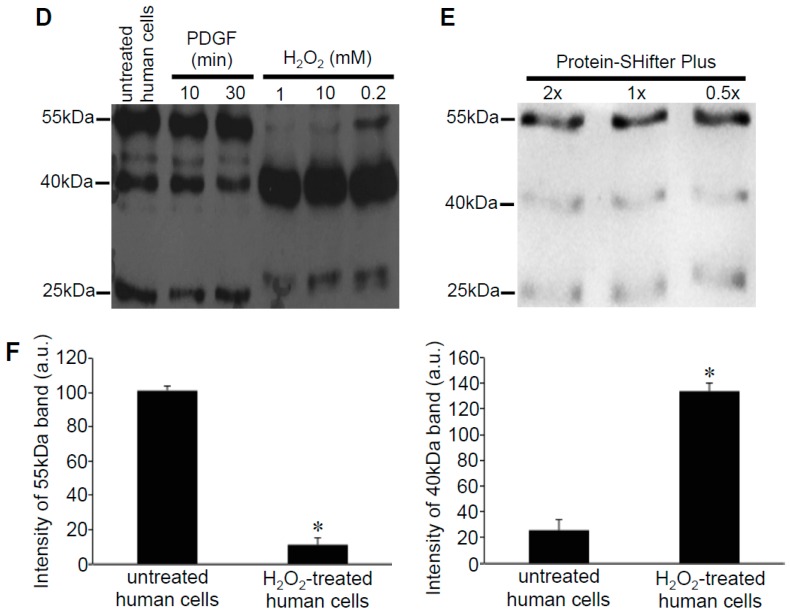
Effects of H2O2 on thiol state of Prx6 in human cells monitored using Protein-SHifter Plus. (A) The principle of the -SulfoBiotics- Protein Redox State Monitoring Kit Plus. A free protein thiol group was labeled with the Protein-SHifter Plus that contained maleimide with a high affinity toward reduced cysteine. After electrophoresis, the large Protein-SHifter Plus moiety was eliminated with UV light, increasing the efficiency of Western blotting and allowing for the detection of specific proteins in the biological samples. (B) Amino acid sequence of human Prx6. Cys47 indicated in red is conserved catalytic cysteine, while Cys91 indicated in blue is non-conserved cysteine. (C) Schematics of the human Prx6 structure with or without Protein-SHifter Plus attached. SOx indicates oxidized cysteine that would not bind to the Protein-SHifter Plus. (D) Human pulmonary artery smooth muscle cells were treated with or without PDGF (10 ng/mL) or H2O2 at indicated concentrations for 15 min. Cell lysates were prepared, incubated with Protein-SHifter Plus, subjected to SDS-PAGE without BME, and immunoblotted with the Prx6 antibody. (E) Untreated cell lysates were incubated with different amounts of Protein-SHifter Plus with 1x being the amount used in all experiments in this study. (F) Bar graphs represent the means ± SEM of the intensities of 55- and 40-kDa bands in arbitrary unit (a.u.) (n = 5). The symbol * represents the value significantly different from the untreated control value at p < 0.05.