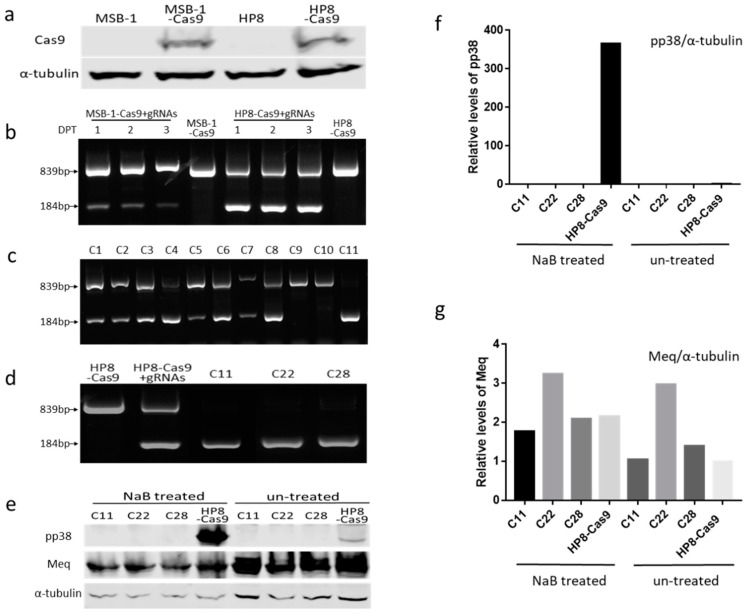
Figure 2.
Deletion of the pp38 gene by CRISPR/Cas9 editing in HP8 cells. (a) Detection of Cas9 expression on single clones of MSB-1 and HP8 cell lines stably expressing Cas9 by western blotting. Cell lysates of MSB-1-Cas9 and HP8-Cas9 along with MSB-1 and HP8 were separated by SDS-PAGE, Western blotted, and probed with anti-Flag antibody, α-tubulin was used as the loading control. (b) PCR amplification of the edited region, using primers NF and CR on the cell lysates of transfected cells at 1, 2, and 3 days post transfection. DPT, days post transfection (c) PCR amplification of the pp38 locus on isolated single cell clones of transfected HP8-Cas9 with two part gRNA system showing the two bands. (d) PCR results of selected clones used for subsequent analysis. The unedited HP8-Cas9 and the mixed population after editing (HP8-Cas9 + gRNAs) were also included. (e) Detection of pp38 expression by western blotting with anti-pp38 monoclonal antibody BD1 and anti-Meq monoclonal antibody FD7 before and after NaB treatment on HP8-Cas9 and the edited clones. For the loading control, the same blot was stripped and re-probed with anti-α-tubulin antibody. (f) and (g) Relative signal intensities of the pp38 (f) and Meq (g) western blot band were quantified using ImageQuant and normalized against the corresponding signal from the α-tubulin band. The signal from untreated control HP8-Cas9 cells was set as 1.