Figure 7. Binding isotherms of selected Hsp16.5 variants to bimane-labeled T4L-L99A.
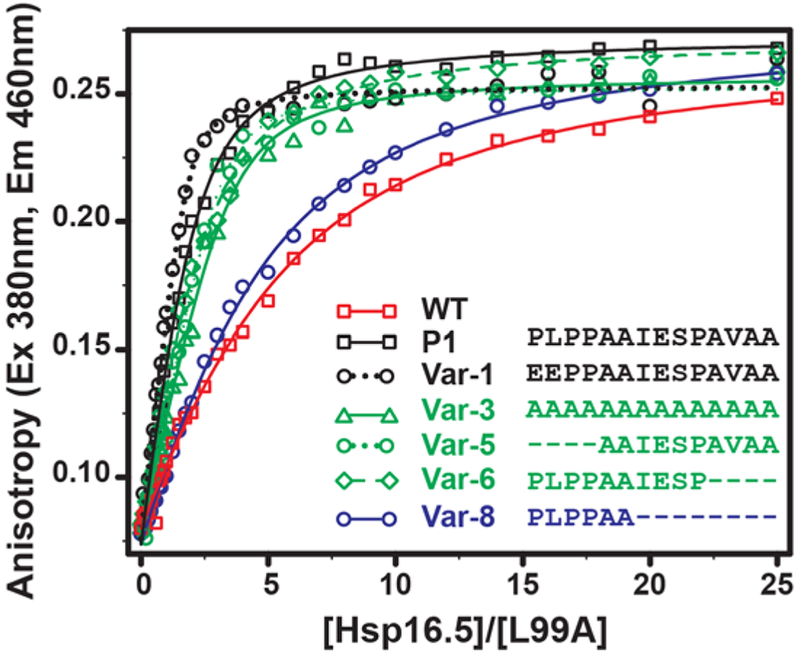
An increase in binding affinity correlates with variants that form large oligomers relative to WT. T4L (5μM) was incubated with increasing molar concentrations (X-axis) of sHSP variants for 2hrs at 37 °C in pH 7.2 buffer, and the fluorescence anisotropy (Y-axis) of the labeled substrate T4L-L99A was measured. The solid lines are non-linear least-squares fits to a single-mode binding model. The KD for each variant is reported in Table S4.
