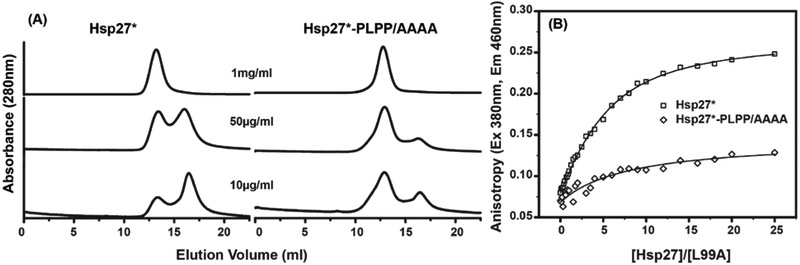
Figure 8. Impact of the PLPP motif on the assembly of Hsp27* oligomers and client protein binding.
A, Concentration-dependent dissociation of the Hsp27* oligomer into the functionally-active dimer is disrupted when the PLPP sequence is substituted with a tetra-alanine peptide. 100μl of the proteins, at the indicated concentrations, were eluted at room temperature from the SEC column at pH 7.2. B, Binding to bimane-labeled T4L-L99A is highly attenuated for Hsp27*-PLPP/AAAA mutant, which correlates with reduced propensity to form the dimeric species. T4L-L99A (5μM) was incubated with increasing molar excess of Hsp27* (X-axis) for 2hrs at 37 °C in pH 7.2 buffer, and the fluorescence anisotropy (Y-axis) of the labeled substrate T4L-L99A was measured. (See also Figure S7)