Figure 3:
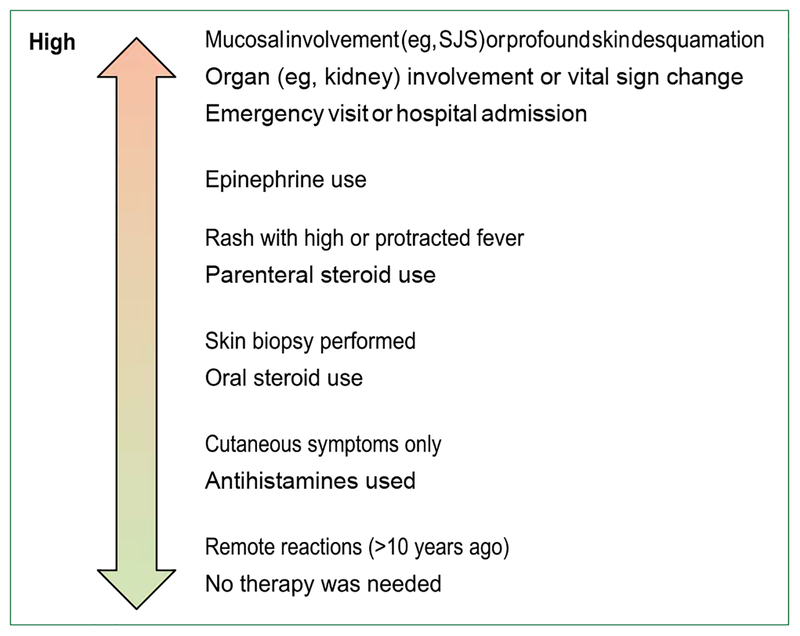
Patient-reported history for risk stratification When limited allergy details are available, patient-reported historical details can be used to distinguish patients at high and low risk. In the case of penicillin allergy, patients with low risk histories are unlikely to be allergic and could be referred on large scales for allergy evaluations. When details are available about the purported reaction, the following questions are important components of the drug allergy history. (1) What were the symptoms? (raised, red, itchy spots with each lesion lasting less than 24 h [hives or urticaria]; swelling of the mouth, eyes, lips, or tongue [angioedema]; blisters or ulcers involving the lips, mouth, eyes, urethra, vagina, or peeling skin [severe type IV HSRs, SCARs]; respiratory or haemodynamic changes [anaphylaxis]; joint pains [serum sickness and serum-sickness like reaction]; organs involvement such as kidneys, lungs, or liver [severe type IV HSRs]). (2) What was the timing of the reaction after taking penicillin [minutes, hours, or days later]? Was it after the first dose or after multiple doses? (3) How long ago did the reaction happen? (4) How was the reaction treated? Was there a need for urgent care or was epinephrine administered? (5) Has the patient tolerated similar medications, such as ampicillin, amoxicillin, or cephalexin since the penicillin reaction?