Figure 2.
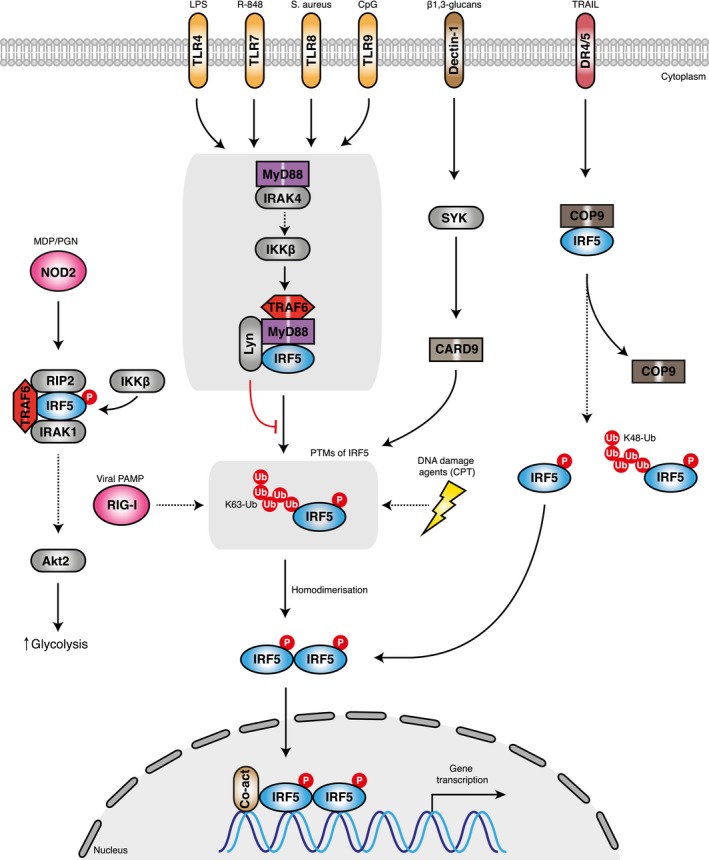
A schematic representation of the proposed mechanisms of IRF5 activation. The stimulation of toll‐like receptors (TLRs) on cell membranes by pathogen‐derived products or RIG‐I‐like receptors (RLRs) in the cytosol by viral PAMPs trigger signalling cascades. IRAK4 regulates the TLR/MyD88 response via TAK1 and IKKβ. Interferon regulatory factor 5 (IRF5) is activated from its latent state by post‐translational modifications that include ubiquitination by the ubiquitin ligase TRAF6 and phosphorylation by IKKβ. These modifications trigger IRF5 homodimerization, translocation to the nucleus and binding to gene promoters along with coactivator proteins (p300). Alternatively, DNA damage agents (CPT) can induce IRF5 phosphorylation and transcriptional activation. Another model proposes that the Dectin‐1‐induced IFN‐B production is mediated by a Syk‐Card9‐IRF5‐dependent pathway. In another model, IRF5 interacts with the COP9 signalosome (CSN). Upon stimulation with agonists such as TRAIL, an unknown kinase phosphorylates IRF5 which leads to its dissociation from CSN. Loss of the CSN–IRF5 interaction leads to K48‐linked ubiquitination of IRF5 and degradation by the ubiquitin–proteasome pathway. Some activated IRF5 migrates to the nucleus and activates target genes. An alternative model suggests that upon NOD2 stimulation IRF5 associates with RIP2, IRAK1, TRAF6 and IKKb to increase glycolysis through Akt2 activation.
