Figure 1.
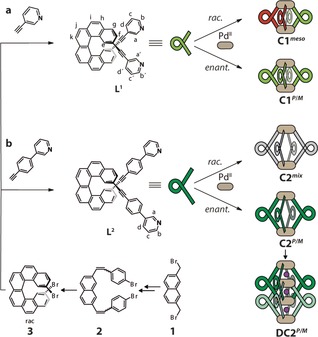
a, b) Synthesis of ligands L1 and L2 from 2,15‐dibromo[6]helicene 3 followed by separation into the P (red) and M (green) enantiomers. The addition of stoichiometric amounts of PdII leads to the quantitative formation of different coordination cages, depending on the enantiomeric composition and length of the ligand. Racemic L1 exclusively gives C1 meso, whereas racemic L2 leads to a statistical mixture of all possible stereoisomers (PPPM/MMMP/PPMM/PMPM/PPPP/MMMM, shown in gray). The enantiopure ligands give the chiral coordination cages C1 P/M and C2 P/M and the interpenetrated dimer DC2 P/M.
