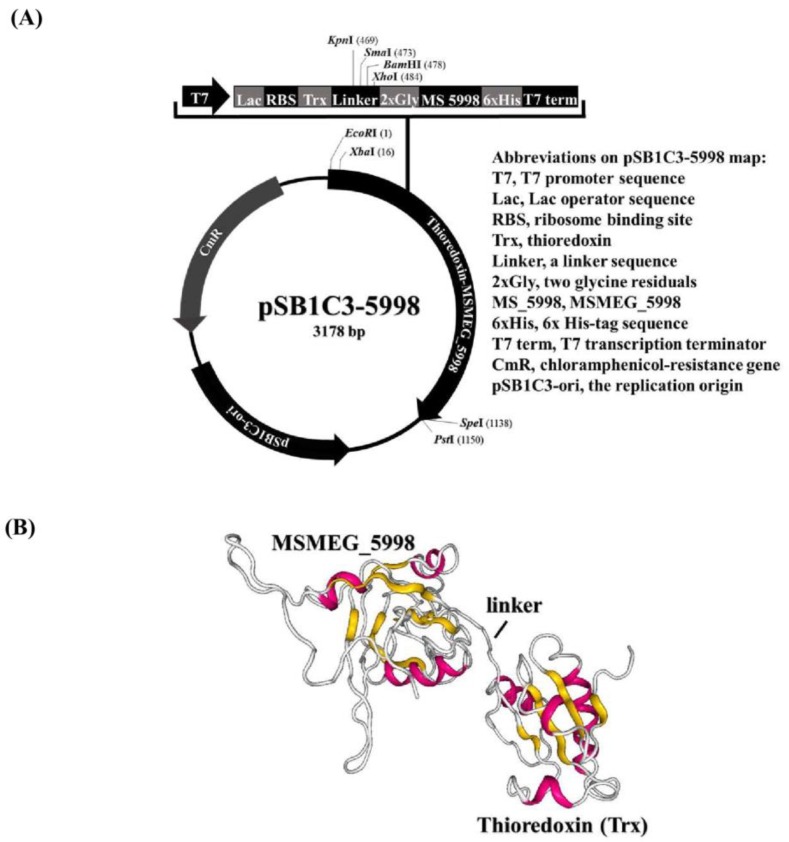
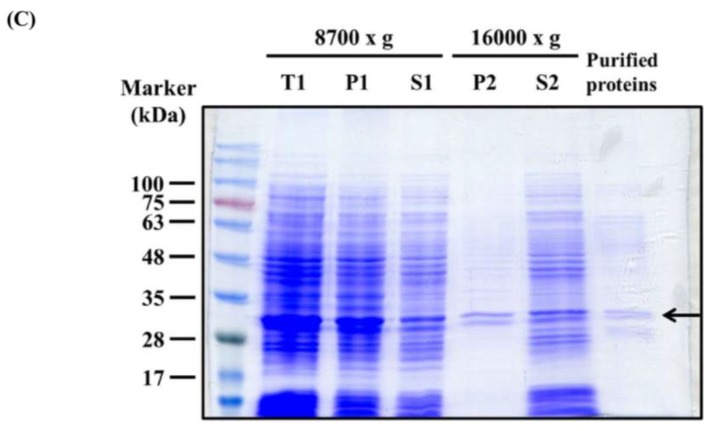
Figure 1.
Thioredoxin-linked (Trx) MSMEG_5998 expression vector and protein production. (A) Schematic diagram of the construction of pSB1C3-5998. The plasmid contains T7 promoter, lac operator, ribosome binding site (RBS), thioredoxin A gene (Thio), a linker with an enterokinase recognition site, MSMEG_5998 (MS 5998) gene [modified sequence from that deposited in the National Center for Biotechnology Information (NCBI) database], 6× His-tag sequence, and T7 terminator (term). (B) The structure of Trx-linked MSMEG_5998 predicted using RaptorX Model. (C) SDS-PAGE analysis of affinity-purified Trx-linked MSMEG_5998. The produced Trx-linked MSMEG_5998 was purified by nickel-chelate affinity chromatography under native conditions. Samples obtained during purification process (lanes 1–5) and the purified enzyme were examined by SDS-PAGE. E. coli cells were broken by sonication (Section 5.3 in Materials and Methods) and the total cell lysates (T1) were centrifuged twice. After the first centrifugation at 8700× g, total lysate was separated into a pellet (P1) and supernatant (S1). S1 was further separated into a pellet (P2) and supernatant (S2) by a second centrifugation, at 16,000× g. The preparation of T1, P1, S1, P2, and S2 factions was described in Materials and Methods (Section 5.3). Protein concentration in the samples was adjusted to the same value. Trx-linked MSMEG_5998 protein was indicated by an arrow.