Figure 2.
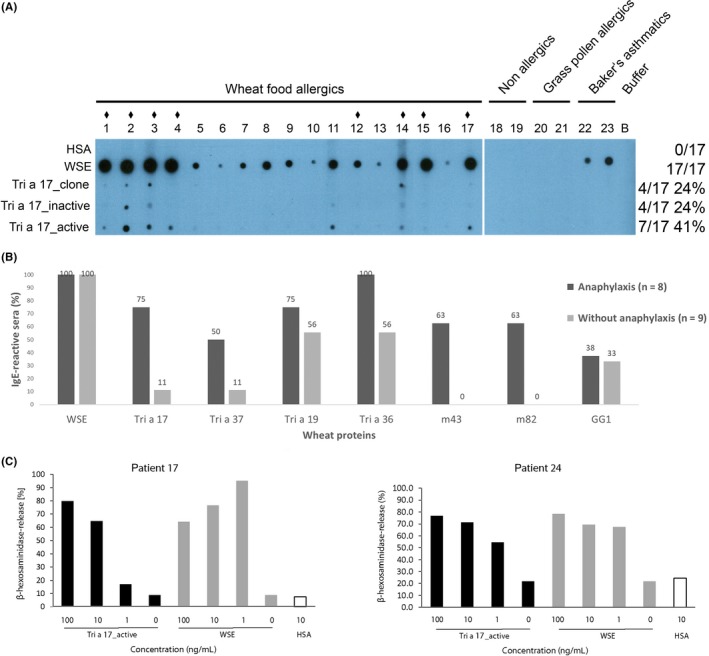
A, IgE reactivity of wheat β‐amylases (Tri a 17_clone, Tri a 17_inactive and Tri a 17_active). Nitrocellulose‐dotted human serum albumin (HSA), wheat seed extract (WSE), Tri a 17_clone and Tri a 17, in its inactive and active form, were tested with sera from wheat food allergic patients (1‐17). For control purposes, sera from non‐allergic individuals (18 and 19), grass pollen allergic patients (20 and 21) baker's asthma patients (22 and 23), or buffer alone (B) were included. Patients with a history of wheat‐induced anaphylaxis are indicated by ♦ above their number. B, IgE reactivity of wheat food allergic patients with and without anaphylaxis. IgE‐binding prevalences (y‐axis: percentage of IgE‐reactive sera) to wheat seed extract and wheat proteins (Tri a 17, Tri a 37, Tri a 19, Tri a 36, m43, m82, GG1) for patients with (black bars) and without anaphylaxis (gray bars). C, Allergenic activity of recombinant β‐amylase Tri a 17_active and wheat seed extract. RBL cells were loaded with serum IgE from Tri a 17_active‐reactive patients and incubated with increasing concentrations of Tri a 17_active (black) or WSE (gray) (100, 10, 1 ng/mL), buffer alone (0 ng/mL) or HSA (white) (10 ng/mL). β‐Hexosaminidase releases are displayed as percentages of total β‐hexosaminidase release on the y‐axes
