Figure 4.
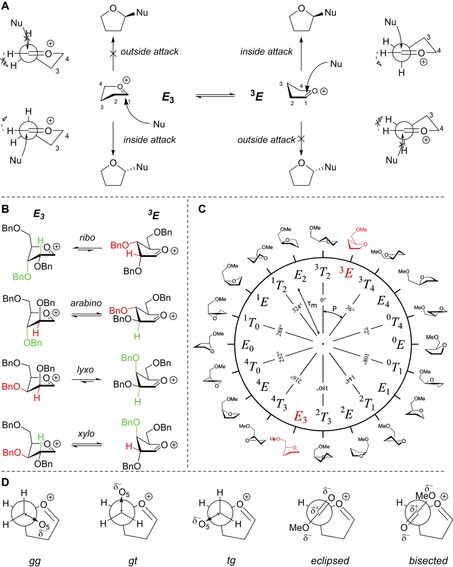
A) The two‐conformer model, visualising the preferential nucleophilic attack from the inside face. Important rotations are denoted by dashed arrows. B) The two principal conformations of the two‐conformer model (E 3–3 E) shown for every carbohydrate configuration, examples taken as their tri‐O‐benzyl‐protected form. The colours indicate the relative preferential orientations for H2 and O3: green is relatively stabilising whereas red is relatively destabilising. C) Pseudo‐rotational circle showing the twenty canonical furanoside structures, with phase‐angles (P) and puckering amplitudes (τ m). D) Possible C4−C5 rotamers: gg, gt and tg for the C5‐OMe oxocarbenium ions, and two rotamers, eclipsed and bisected, for the C4‐CO2Me oxocarbenium ions.
