Table 3.
Hydrothiolation of Various 1,3-Dienesa
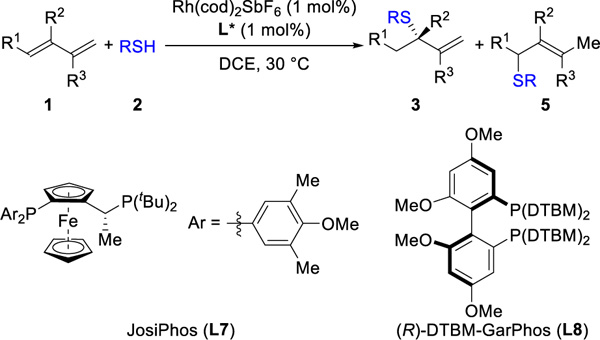 |
| (A) Hydrotholation of unsymmetric dienesb |
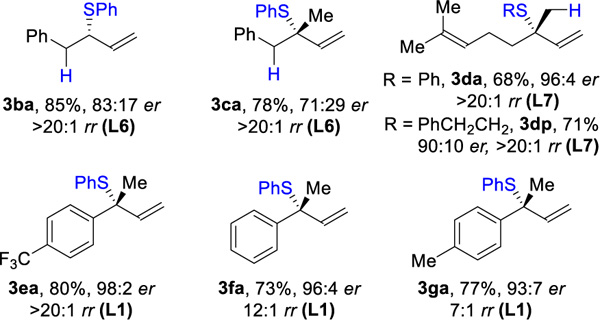 |
| (B) Hydrotholation of feedstock dienes(> 20:1 rr) |
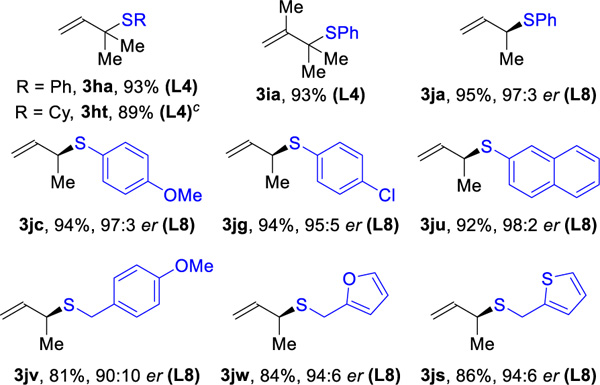 |
Reaction conditions: 1 (0.4 mmol), 2 (0.2 mmol), Rh(cod)2SbF6 (1 mol%), L (1 mol%), DCE (0.4 mL), 30 °C, 5 h. Isolated yields. Ligand used in parentheses. Regioselectivity ratio (rr) is the ratio of 3 to 5, which is determined by 1H NMR analysis of reaction mixture. Enantioselectivity de-termined by chiral SFC.
Using Rh(cod)2SbF6 (5 mol%), L (5 mol%), 15 h.
13:1 rr
