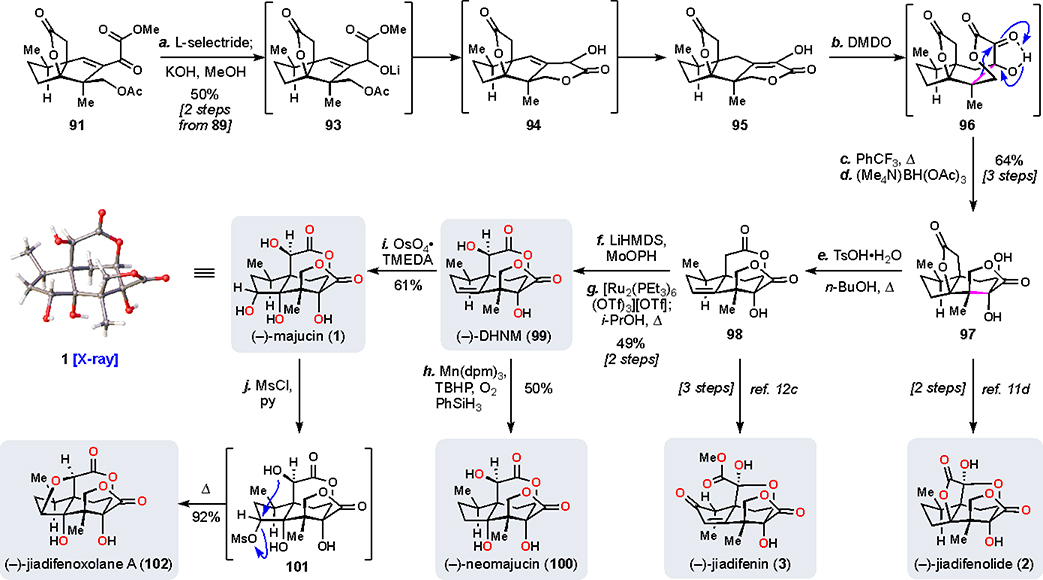
Scheme 8.
Synthesis of the Majucinoidsa
aReagents and conditions: (a) L-selectride (1.2 equiv), THF, −78 °C, 30 min then KOH (10.0 equiv), MeOH, 0 °C, 30 min, 50% (two steps from 89); (b) DMDO (1.5 equiv), 12 h; (c) PhCF3, 170 °C, 2 h; (d) Me4NBH(OAc)3 (7.0 equiv), MeCN/AcOH (3:1), −40 °C, 16 h, 64% (three steps); (e) TsOH•H2O (2.2 equiv), n-BuOH, 150 °C, 26 h, 71%; (f) LHMDS (3.0 equiv), MoOPH (5.0 equiv), THF, −78 → 0 °C, 2.5 h, 65%; (g) [Ru2(PEt3)6(OTf)3](OTf) (0.1 equiv), NMM (0.2 equiv), TFE/dioxane (1:1), 120 °C, 18 h then i-PrOH (3.0 equiv), 120 °C, 5 h, 75%; (h) Mn(dpm)3 (0.2 equiv), TBHP (1.5 equiv), PhSiH3 (2.0 equiv), O2 (1 atm), DCM/i-PrOH (4:1), 0 °C, 20 h, 50%; (i) OsO4•TMEDA (1.0 equiv), DCM, −78 → 0 °C, 2 h then NaHSO3 (10.0 equiv), H2O, 16 h, 61%; (j) MsCl (5.0 equiv), pyr. (10.0 equiv), DCE, rt →80 °C, 15 h, 92%. DMDO = dimethyldioxirane, LHMDS = lithium bis(trimethylsilyl)amide, MoOPH = oxodiperoxymolybdenum(pyridine)(hexamethylphosphoric triamide), dpm = dipivaloylmethane.