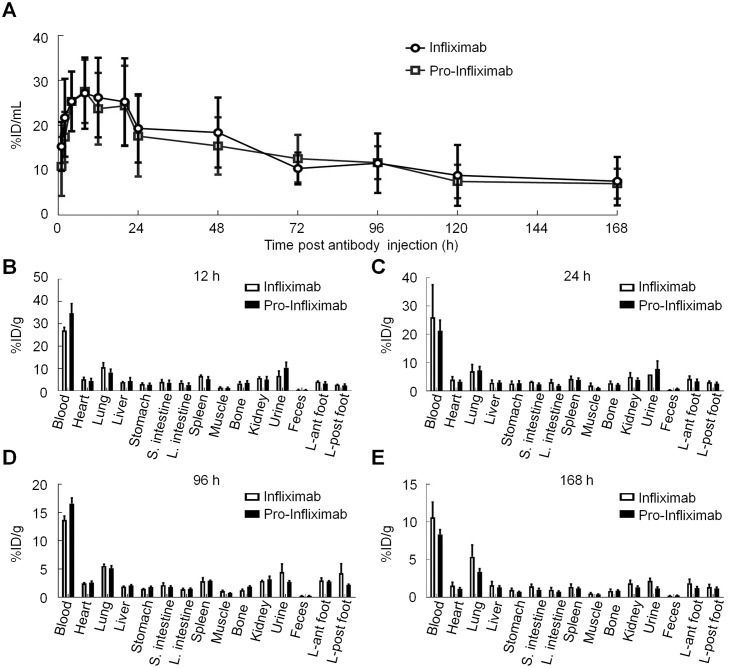
Fig 4. Evaluation of the PK properties and biodistribution of pro-Infliximab and Infliximab.
The DBA/1J mice were intraperitoneally injected with 131I-labeled Infliximab (○) or 131I-labeled pro-Infliximab (□). The blood was collected at different time and the radioactivity was detected via the γ counter to measure the (A) PK properties of 131I-labeled Infliximab and 131I-labeled pro-Infliximab. The biodistribution of 131I-labeled Infliximab and 131I-labeled pro-Infliximab in several tissues and organs in DBA/1J mice at 12 h (B), 24 h (C), 96 h (D), and 168 h (E) after injection. PK properties and biodistribution are expressed as radioactivity %ID/g. Error bar: standard error of triplicate determinations. Underlying data can be found in S1 Data. DBA/1J, dilute brown non agouti; %ID/g, percent injected dose per gram; PK, pharmacokinetic.