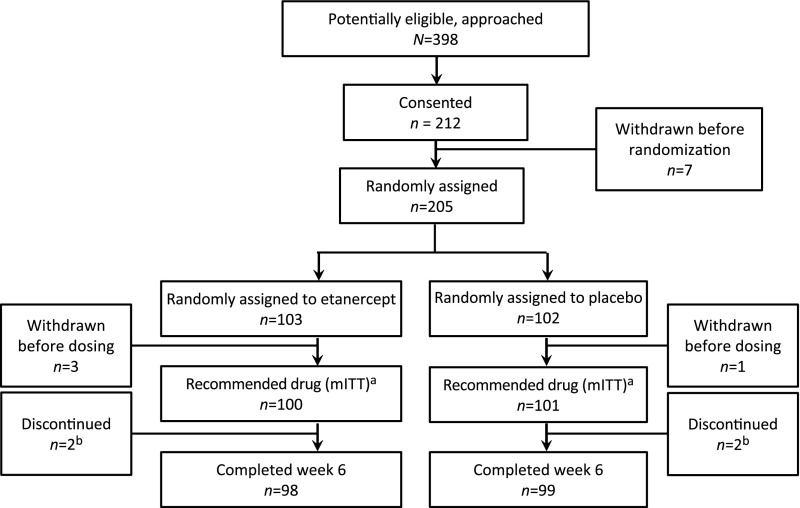
FIGURE 1.
Approach, consent, randomization, and follow-up of participants. Four randomly assigned patients withdrew before dosing. A total of 201 patients received study medication; 4 received at least an initial dose but did not receive a second or third dose. They were included in the mITT analyses. Patients received “drug” either etanercept or placebo. a One subject was randomly assigned to etanercept but received the placebo. b In the etanercept arm, 1 subject was determined to have measles and not KD, and the parents of the other refused to attend the final follow-up visit. In the placebo arm, 2 subjects’ parents refused further dosing.