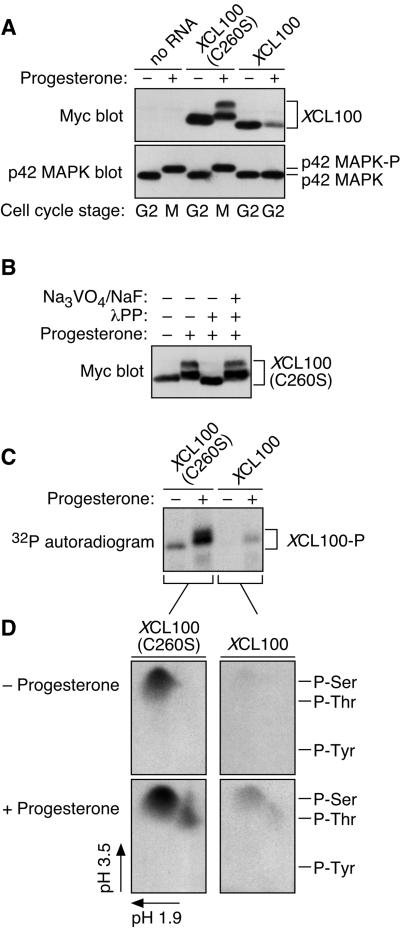
Figure 2.
Cell cycle-regulated phosphorylation of XCL100. (A) Lysates from unstimulated and progesterone-stimulated oocytes expressing Myc-tagged XCL100 or XCL100(C260S) were immunoblotted with 9E10 (to detect XCL100 proteins) or DC3 (to detect p42 MAPK) followed by ECL. (B) Lambda protein phosphatase treatment of XCL100(C260S) from mature oocytes. Lysates from XCL100(C260S)-expressing oocytes were treated with (+) or without (−) lambda protein phosphatase (2 U/μl for 30 min then an additional 2 U/μl for 60 min) in the absence (−) or presence (+) of sodium orthovanadate (Na3VO4, 2 mM final) and sodium fluoride (NaF, 10 mM final). XCL100(C260S) proteins were visualized by 9E10 immunoblotting and ECL. (C) Radiolabeling of XCL100 in vivo. Stage VI oocytes, microinjected with mRNA (50 ng) encoding XCL100 or XCL100(C260S), were immediately transferred to phosphate-free OR2 containing 1 mCi/ml [32P]orthophosphate and incubated 3 h later with (+) or without (−) progesterone. Oocytes (10 per sample) were washed and collected after 7 h, and the radiolabeled XCL100 proteins were immunoprecipitated with 9E10 and visualized by autoradiography. Total radiolabel incorporation was comparable in all sets of oocytes (our unpublished observations). (D) Phosphoamino acid analysis of 32P-labeled XCL100. The radiolabeled, immunoprecipitated XCL100 proteins were excised from the blotting membrane, subjected to partial acid hydrolysis, and analyzed by two-dimensional thin-layer electrophoresis at pH 1.9 in the first dimension and pH 3.5 in the second dimension. The radiolabeled amino acids were visualized by autoradiography, and their identities were confirmed by comparison to ninhydrin-stained standards (indicated on the right). XCL100-P, phosphorylated XCL100.