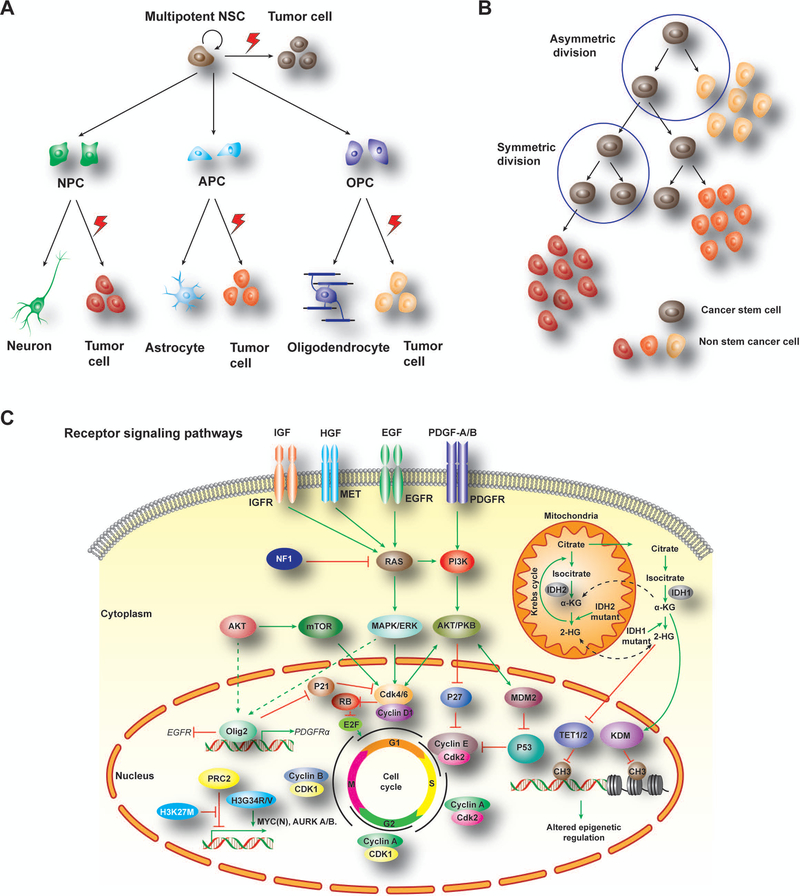
Figure 1. Cells of origin and tumor-driving pathways in brain tumors.
A) Multipotent NSCs have capability to self-renew and differentiate into different fate-restricted progenitors. Under genetic and epigenetic alterations, these progenitor cells could be transformed into malignant tumors including gliomas from NSC, OPC or APC as well as medulloblastomas from NPCs.
B) A hierarchical model for tumor cell evolution and plasticity to generate distinct tumor-forming cells and tumor heterogeneity gained after tumor initiation.
C) Malignant transformation of GBM is the result of different driver pathway alterations. RTK signaling pathway activation mediated by extracellular signal (growth factors, cytokines, and hormones) promotes cell cycling for tumorigenesis. Besides, point mutation of histone 3 (H3K27M, H3G34R/V) and up-regulation of oncogenic factors, like MYC(N) and AURK, which lead to tumor cell proliferation. Moreover, IDH mutations later Krebs cycle in mitochondria, which further alter activity of downstream epigenetic regulators, including TET1/2 and KDM, leading to methylation alterations for DNA and nucleosome states susceptible to for tumorigenesis. GBM, glioblastoma; NSCs, neural stem cells; NPC, neuron progenitor cell; APC, astrocyte progenitor cell; OPC, oligodendrocyte progenitor cell; RTK, receptor tyrosine kinase; MYC, myelocytomatosis viral oncogene; AURK, aurora kinase; IDH, Isocitrate dehydrogenase; TET, Tet oncogene; KDM, lysine(K)-specific demethylase.