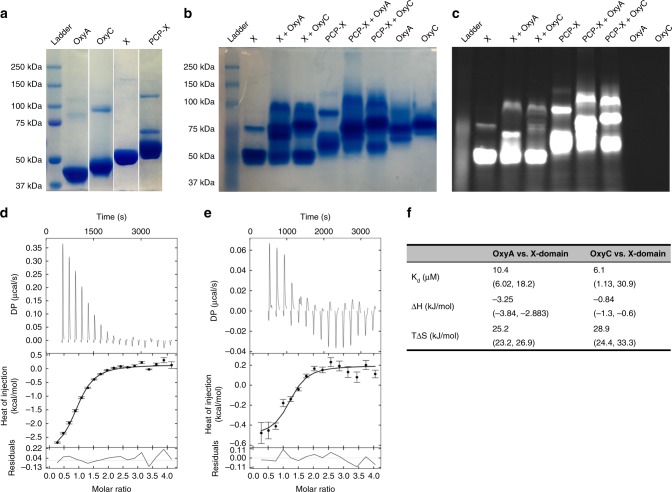
Fig. 4.
Interaction of OxyAkis and OxyCkis with the X-domain from the kistamicin NRPS. a SDS-PAGE of OxyAkis (44.9 kDa), OxyCkis (48.8 kDa), the Xkis-domain (52.0 kDa) and PCP-Xkis (60.2 kDa). Native PAGE of the Xkis-domain and PCP-Xkis didomain in isolation and after co-incubation with OxyAkis and OxyCkis visualised both with Coomassie blue stain (b) and using UV visualisation (c, following prior FITC labelling of the Xkis-domain and the PCP-Xkis didomain). d Representative ITC isotherm data for interactions between (d) OxyAkis and (e) OxyCkis with the Xkis-domain. The upper panels represent baseline-corrected power traces; by convention, negative power corresponds to exothermic binding. The middle panels represent the integrated heat data fitted to the single binding sites model in SEDPHAT58, with figures produced using GUSSI59. The bottom panels show the residues of the fit with error bars are standard error in the integration of the peaks as calculated by NITPIC (n = 1)57. f Binding affinities of OxyAkis and OxyCkis with the Xkis-domain. Affinities were determined by ITC at 25 °C with stirring at 300 rpm. 68.3% confidence intervals (1 Std. Dev.) are given in parentheses for Ka and Kd as calculated from a single titration. Source data are provided as a Source Data file