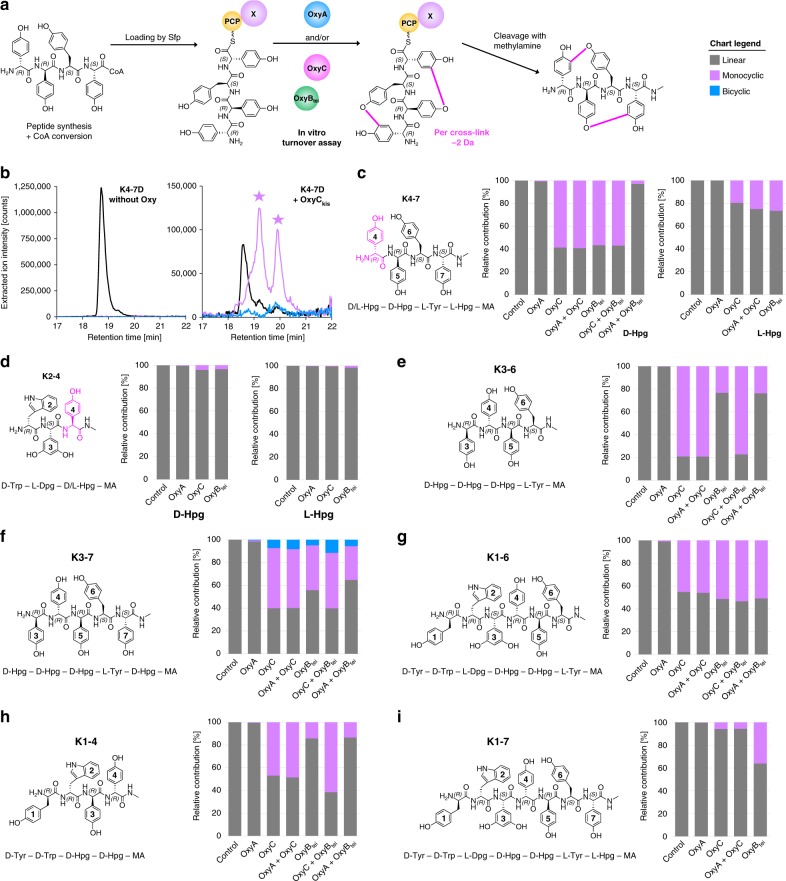
Fig. 7.
In vitro reconstitution of kistamicin Oxy enzymes. a Schematic illustration of in vitro reaction: kistamicin peptides were synthesised on hydrazine resin and converted into their CoA thioesters that were then loaded into the PCP-Xkis didomain by the phosphopantetheinyl transferase Sfp. After incubation with OxyAkis, OxyCkis and/or OxyBtei (different combinations), the peptide was cleaved from the PCP by the addition of methylamine and subsequently analysed by HPLC-MS. b Example shown for the peptide K4-7D: in the control reaction, only linear peptide is detected. Reactions containing OxyCkis led to the formation of several monocyclic products and traces of bicyclic compounds. Structures of synthesised peptide probes and turnover results of c K4-7 (4-D) (left) and (4-L) (right), d K2-4 (4-D) (left) and (4-L) (right), e K3-6, f K3-7, g K1-6, h K1-4 and i K1-7. Detection of linear peptide mass is indicted in dark grey, of monocyclic compound in purple and bicyclic compound in blue. Source data are provided as a Source Data file