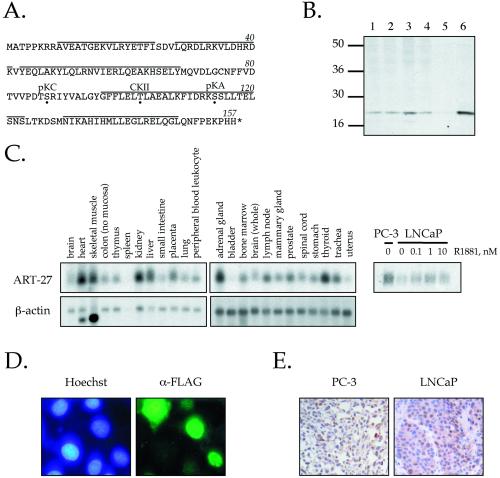
Figure 1.
Cloning and characterization of ART-27. (A) Amino acid sequence of ART-27. The amino acid sequence of ART-27 is shown with an asterisk, representing the stop codon. Potential phosphorylation sites for protein kinase C (pKC), casein kinase II (CKII), and protein kinase A (pKA) are marked by a dot below the target residue. Lines above the sequence represent predicted α-helical regions as determined by Chou-Fasman and GarnierOsguthorpe-Robson algorithms. (B) Expression of ART-27 protein. Equal amounts (50 μg) of nuclear extracts prepared from HeLa cells (lane 1), treated with TPA for 2 h (lane 2), serum-starved and stimulated with serum for 2 h (lane 3), PC-3 cells (lane 4), and whole cell extracts from COS1 cells transfected with pcDNA3 (lane 5) or pcDNA3:ART-27 (lanes 6) were analyzed by immunoblotting with affinity-purified anti–ART-27 antibody. No ART-27 immunoreactivity is observed with preimmune serum (Markus, Taneja, Logan, Li, Ha, Hittelman, Rogatsky, and Garabedian, unpublished results). (C) ART-27 mRNA expression in human tissues and prostate cancer cell lines. A human multiple tissue Northern blot (CLONTECH: human 12-lane MTN Blot I and II) containing 2 μg of poly(A+) mRNA from the indicated tissues was hybridized with 32P-labeled probes corresponding to ART-27 (top) and β-actin (bottom). Total RNA was extracted from PC-3 and LNCaP cells cultured in the absence or presence of the indicated doses of androgen (R1881) for 72 h. Equal amounts of RNA were separated on denaturing formaldehyde-agarose gels (see MATERIALS AND METHODS), transferred to a Duralon nylon membrane, and hybridized to 32P-labeled cDNA probes corresponding to ART-27 (right). Equal loading for each lane was determined by ethidium bromide staining of the 28S rRNA (Markus, Taneja, Logan, Li, Ha, Hittelman, Rogatsky, and Garabedian, unpublished results). (D) ART-27 subcellular localization. HeLa cells were transfected with an FLAG–ART-27 expression construct, fixed, permeabilized, and incubated with an anti-FLAG primary antibody and a corresponding fluorescein-conjugated secondary antibody, and the DNA in nucleus was stained with Hoechst dye H334211. Cells were visualized using a Zeiss Axioplan 2 fluorescence microscope. No signal above background was observed when the primary antibody was omitted (Markus, Taneja, Logan, Li, Ha, Hittelman, Rogatsky, and Garabedian, unpublished results) or in nontransfected cells. Note that the FLAG–ART-27 fluorescence is localized predominantly to the nucleus. (E) Expression pattern of endogenous ART-27 in human PC-3 and LNCaP prostate cancer cell xenografts. Immunohistochemical analysis of paraffin-embedded PC-3 (left) and LNCaP (right) human prostate cancer cell xenografts hybridized with affinity-purified ART-27 antibody is shown (200×). Immunoreactivity, seen as brown staining within the nuclei of these cells, is blocked by coincubation of the antibody with the ART-27 peptide immunogen (Markus, Taneja, Logan, Li, Ha, Hittelman, Rogatsky, and Garabedian, unpublished results).