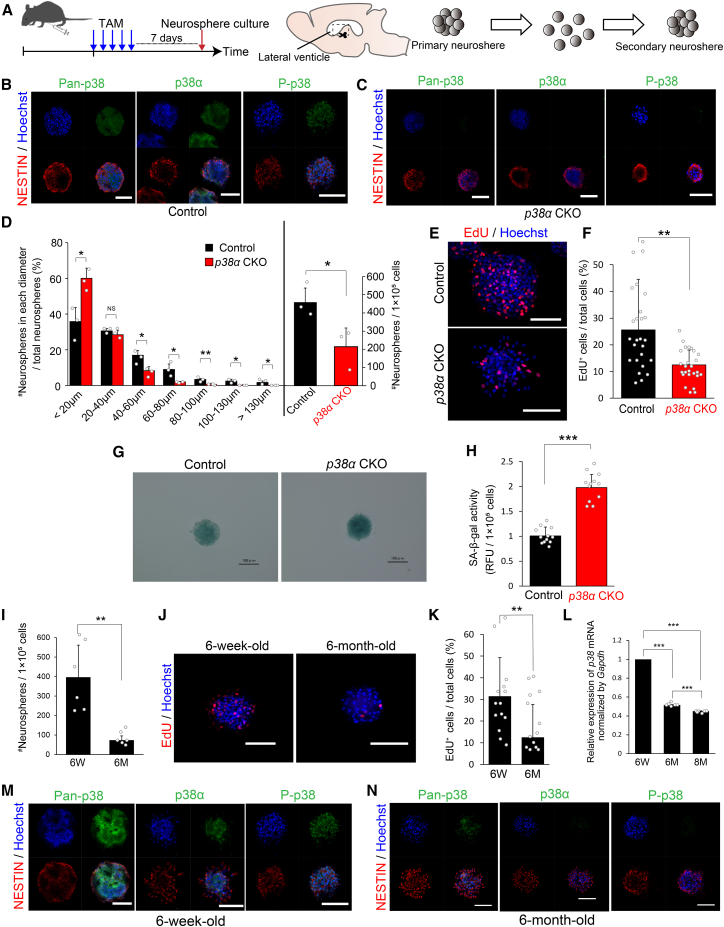
Figure 4.
Impaired Proliferation of the NS/PCs of p38α CKO Mice and Aged Mice In Vitro
(A) Strategy for inducible conditional deletion of p38α in p38αflox/flox;Tg (Nes-cre/ERT2) mice for an in vitro NPC proliferation assay via neurosphere formation. Four-week-old mice were injected i.p. with TAM for 5 consecutive days and processed for neurosphere formation 7 days after injection.
(B and C) Representative confocal images of pan-p38, p38α, P-p38 (all green), and NESTIN (red) reactivities in neurospheres from control (B) and p38α CKO mice (C) (6 weeks old). Nuclei were counterstained with Hoechst (blue). Scale bars, 100 μm.
(D) Distribution of the different sizes of secondary neurospheres and their total numbers between control and p38α CKO mice (n = 3 independent cultures). The p38α CKO neurospheres were relatively smaller and less expandable.
(E) Representative confocal images of EdU+ cells (red) in control and p38α CKO neurospheres. EdU (10 μM) was administered 30 min before fixation of neurospheres. Nuclei were stained with Hoechst (blue). Scale bars, 100 μm.
(F) EdU incorporation into the neurospheres was significantly reduced by p38α deletion (n = 6 independent cultures; at least 1,500 cells were analyzed).
(G) SA-β-gal staining of control and p38α CKO neurospheres in blue. The color is darker in the p38α CKO neurosphere than in the control neurosphere. Scale bars, 100 μm.
(H) Quantitative analysis of SA-β-gal activity. SA-β-gal activity was significantly increased in p38α CKO neurospheres compared with that in control neurospheres (n = 3 independent cultures). RFU, relative fluorescence units.
(I) The number of secondary neurospheres from aged mice (6 months old) was significantly lower than that of young mice (6 weeks old) (n = 6 independent cultures).
(J) Representative confocal images of EdU+ cells (red) in neurospheres from young and aged mice. EdU labeling was performed as described. Nuclei were counterstained with Hoechst (blue). Scale bars, 100 μm.
(K) EdU incorporation in the neurospheres was significantly reduced in aged mice (n = 6 independent cultures; at least 600 cells were analyzed).
(L) Quantitative analysis of the p38 mRNA expression in secondary neurospheres. The relative expression of p38 mRNA decreased with age (n = 6 independent experiments).
(M and N) Significant reduction of p38 expression in the neurospheres from aged mice. Pan-p38, p38α, and P-p38 immunoreactivities (green) were barely detected in neurospheres from aged mice (N), whereas all of these immunoreactivities were clearly detected in the neurospheres from young mice (M). There was no significant difference in NESTIN expression (red) between neurospheres from young and aged mice. Nuclei were stained with Hoechst (blue). Scale bars, 100 μm.
Statistical analysis was performed with an unpaired two-tailed Student's t test (D, F, H, I, and K) or one-way ANOVA, Tukey-Kramer post hoc test (L). Values in the bar graphs represent the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. NS, not significant; 6W, 6 weeks old; 6M, 6 months old, 8M, 8 months old; TAM, tamoxifen.