Figure 4.
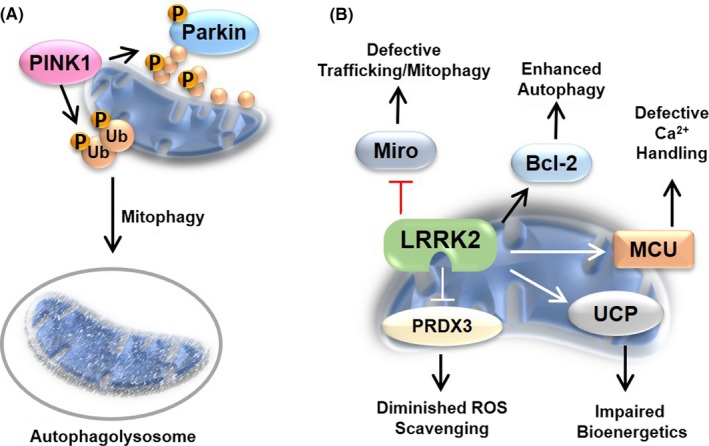
Parkinson's disease‐related protein kinases impact mitochondrial physiology. PD is associated with mitochondrial dysfunction, and numerous proteins implicated in familial PD or disease pathogenesis exhibit mitochondrial localization or influence organelle physiology. (A) Loss‐of‐function mutations in PINK1 are found in familial PD patients. PINK1 accumulates at the OMM following mitochondrial depolarization leading to its activation by autophosphorylation. An activated PINK1 can then phosphorylation ubiquitin on Ser65 (Ub—orange circles) and activate/recruit Parkin to stress, aged, or damaged mitochondrial to facilitate their elimination by mitophagy. (B) Mutations in LRRK2 increase kinase activity and occur in both familial and sporadic PD cases. Increased LRRK2 activity can be linked to changes in autophagic flux through the manipulation of Bcl‐2 and inhibition of Miro, whereby LRRK2 can interact with and sequester Miro preventing turnover and trafficking of damaged organelles. LRRK2 mutants, such as G2019S, are also associated with changes in Ca2+ homeostasis through perturbation of mitochondrial calcium uniporter (MCU) and decreased oxidative stress tolerance by phosphorylation‐dependent impairment of PRDX3. LRRK2 variants are found to affect bioenergetics by inhibiting complex I (not shown) and increasing UCP abundance and stability. The kinases represent direct effectors of PD‐induced mitochondrial dysfunction
