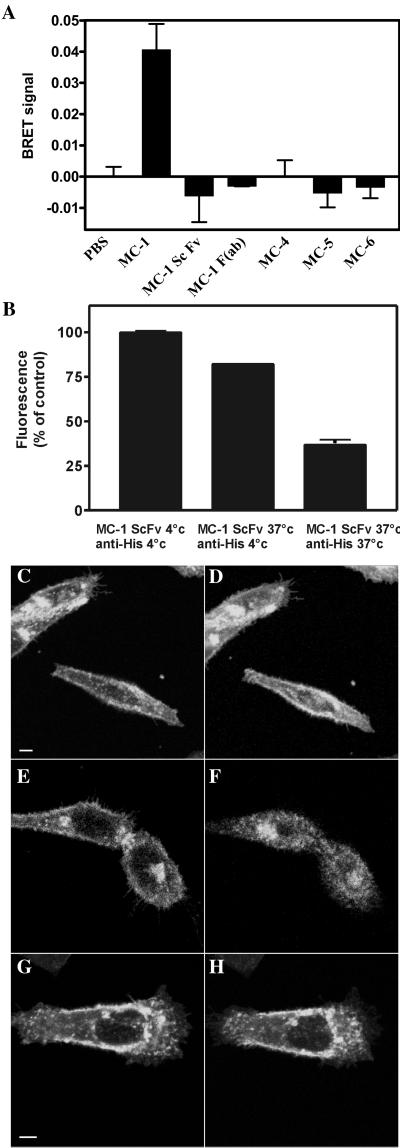
Figure 6.
Receptor oligomerization are involved in MC-1-induced CCR5 endocytosis. (A) 293T cells expressing CCR5-luc and CCR5-YFP were incubated with coelenterazine, and the luminescence and the fluorescence signals were quantified before and after the addition of the indicated mAb. The BRET ratio was defined as [(emission at 485 nm ± 20)/(emission at 530 nm ± 25)] − [(emission at 485 nm ± 20)/(emission at 530 nm ± 25)] for CCR5-luc expressed alone in the same experiments. Variation in BRET signal compared with baseline (addition of buffer alone) after 10 min of incubation with antibodies is displayed. All experiments were repeated at least twice with similar results. All points were performed in triplicates (error bars: SEM). (B) CHO cells expressing CCR5 were incubated with 3 μg/ml ScFv-MC-1 for 45 min at 4 or 37°C, washed twice with cold PBS, and incubated with anti-His at 4 or 37°C as indicated. Surface expression of CCR5 was measured by flow cytometry with PE-labeled anti-mouse antibody. Fluorescence was normalized for cells incubated with ScFv MC-1 and anti-His both at 4°C (100%), and for background fluorescence (0%). (C–H) Confocal microscopy. Cells expressing CCR5-GFP were incubated with 10 μg/ml ScFv-MC-1 at 37°C and are shown before (C) and 45 min after the incubation (D). Cells were then washed three times with buffer (E) and incubated with an anti-polyhistidine antibody (anti-His, 4 μg/ml) for 45 min (F). Videos of the experiment described in this figure are available in the online version of this article. Anti-His antibody alone did not result in CCR5-GFP endocytosis after 45 min of observation (G and H). All experiments were at least repeated twice with similar results.