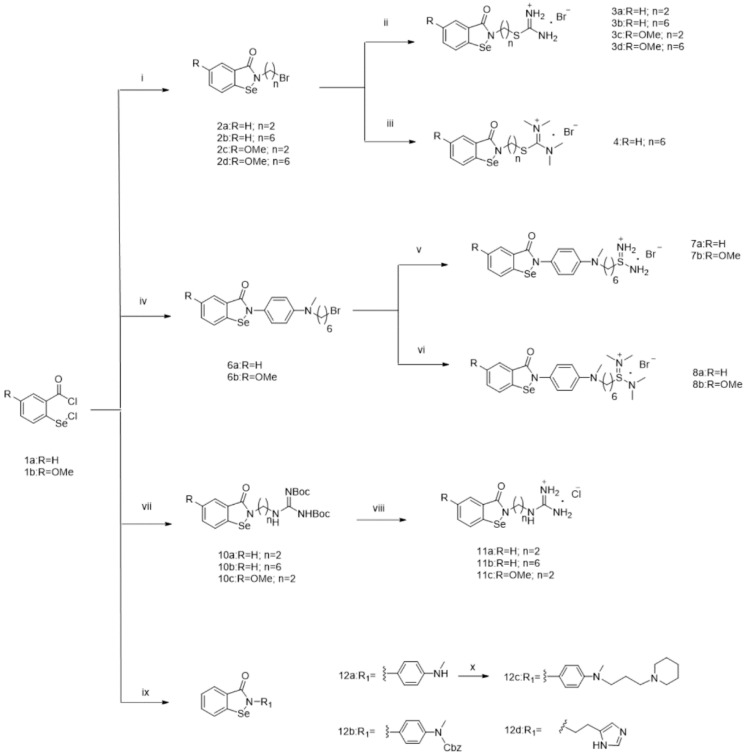
Scheme 1.
Reagents and conditions: (i) NH2(CH2)nBr, Et3N, THF, rt, 5 h, 50–70%; (ii) thiourea, THF, 70 °C, overnight, 40–60%; (iii) tetramethylthiourea, THF, 70 °C, overnight, 55%; (iv) N1-(6-bromohexyl)-N1-methylbenzene-1,4-diamine, Et3N, THF, rt, 5 h, 35–37%; (v) thiourea, THF, 70 °C, overnight, 45–53%; (vi) tetramethylthiourea, THF, 70 °C, overnight, 45–55%; (vii) 9a~b, Et3N, THF, rt, 5 h, 50–70%; (viii) HCl, DCM, 3 h, 90–95%; (ix) R1NH2, Et3N, THF, rt, 5 h, 50–65%; (x) 1-(3-bromopropyl) piperidine, K2CO3, THF, rt, overnight, 40%.