Table 1. Optimization of the reaction conditions a .
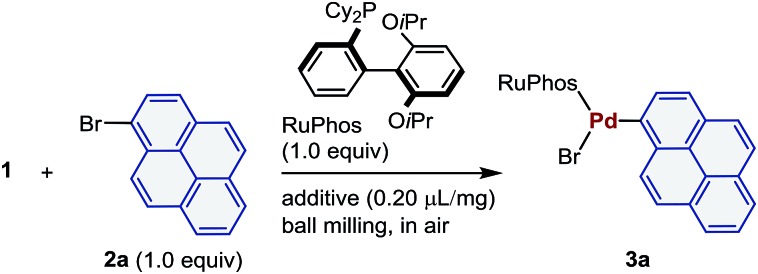
| ||||
| Entry | LAG | Milling frequency (Hz) | Time (min) | NMR yield b (%) |
| 1 | None | 30 | 30 | 40 |
| 2 | None | 30 | 60 | 43 |
| 3 | THF | 30 | 30 | 71 |
| 4 | Toluene | 30 | 30 | 61 |
| 5 | CH3CN | 30 | 30 | 71 |
| 6 | Dioxane | 30 | 30 | 64 |
| 7 | DMF | 30 | 30 | 64 |
| 8 | Et2O | 30 | 30 | 66 |
| 9 | DMSO | 30 | 30 | 55 |
| 10 | Pentane | 30 | 30 | 58 |
| 11 | Cyclohexane | 30 | 30 | 47 |
| 12 | MeOH | 30 | 30 | 48 |
| 13 | THF | 25 | 30 | 78 e |
| 14 c | THF | 25 | 30 | 55 |
| 15 d | THF | 25 | 30 | 67 |
| 16 | THF | 25 | 10 | 71 |
aConditions: 1 (0.05 mmol), 2a (0.05 mmol), RuPhos (0.05 mmol), and the LAG additive (0.2 μL mg–1) in a stainless-steel ball-milling jar (1.5 mL) with a stainless-steel ball (3 mm).
bDetermined by 1H NMR analysis of the crude reaction mixture using an internal standard.
cTwo stainless-steel balls (3 mm) were used.
dThree stainless-steel balls (3 mm) were used.
eIsolated yield.
