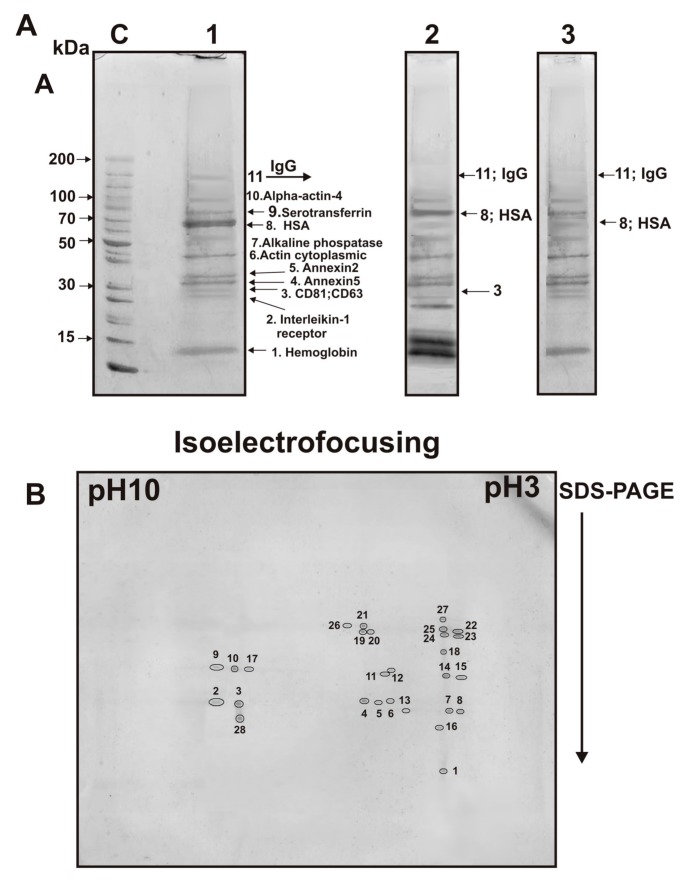
Figure 6.
SDS-PAGE analysis of proteins of the exo-3 preparation after its gel filtration before (lane 1) and after treatment with dithiothreitol (lane 2), as well as after trypsin treatment (lane 3) (A). Molecular weight markers (lane C). After DTT treatment, the molecular weight of HSA increases due to the reduction of disulfide bonds (lane 2), and the band corresponding to IgG disappears due to the formation of free heavy and light chains. Treatment with trypsin leads to the hydrolysis of HSA, IgGs, CD63, and CD81, but not other proteins of this preparation (lane 3, A). Two-dimensional gel electrophoresis of exo-3 proteins after its isolation by gel filtration (B). The proteins were first separated using isoelectric focusing and then by SDS-PAGE. Twenty-eight protein spots stained with Coomassie R-250 correspond to: hemoglobin subunits (spot 1), annexin A2 (2, 3, 4, and 28), annexin A5 (5, 6, and 7), annexin A1 (8), actin cytoplasmic (9, 10, 11, 12, 13, 14, and 15), light (16) and heavy (17 and 18) chains of immunoglobulins, alkaline phosphatase (19, 20, 21, 22, and 23), HSA (24 and 25), and serotransferrin (26, 27).