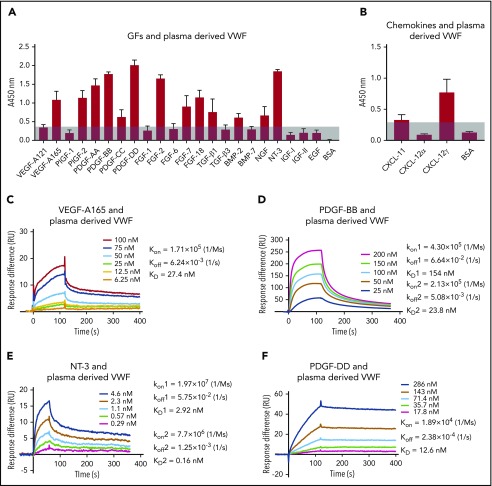
Figure 2.
Human plasma-derived VWF binds promiscuously to GFs with high affinity. VWF binding to (A) GFs and (B) chemokines were measured by ELISA. A450 nm represents absorbance at 450 nm. Signals from VEGF-A121 served as a baseline, and bovine serum albumin (BSA) served as a negative control (n = 4; data are mean ± standard deviation [SD]). Affinity (KD values are shown) of VWF against (C) VEGF-A165, (D) PDGF-BB, (E) NT-3, and (F) PDGF-DD was measured by surface plasmon resonance (SPR). SPR chips were functionalized with VWF (∼2000 resonance units [RU]), and the individual GF was flowed over the chips at indicated concentrations. Curves represent the specific responses (in RU) to VWF obtained. Experimental curves were fitted with (C,F) 1:1 Langmuir fit model and (D-E) heterogeneous ligand-parallel reactions binding. Binding kinetics values (dissociation constants [KD] and rate constants [kon and koff]) determined from the fitted curves are shown.