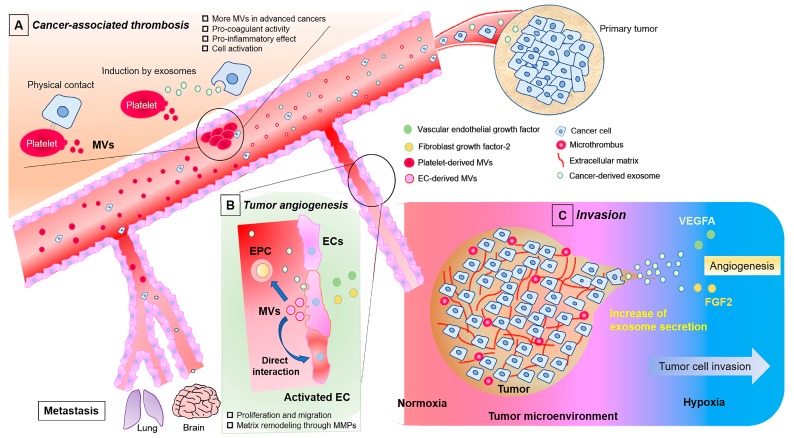
Figure 1.
Involvement of ECs, platelets, and EVs in cancer progression, including angiogenesis, invasion, and metastasis. EVs play important roles in these processes through their function of cell-to-cell communication. Cancer cells develop favorable microenvironments by promoting angiogenesis and procoagulant and proinflammatory activities via the secretion of exosomes and hypoxia-induced growth factors. Platelets are activated via physical contact with cancer-derived exosomes, thereby resulting in increased secretion of platelet-derived microvesicles and the subsequent development of coagulation (A). ECs are activated by VEGF-A and FGF-2 released from cancer cells under hypoxic conditions and by circulating cancer-derived exosomes. Activated ECs shed microvesicles and activate other ECs via the microvesicles and direct interaction (B). Hypoxia is involved in tumorigenesis. A hypoxic microenvironment created by cancer cells triggers the proangiogenic pathway that involves the cancer-derived exosomes secretion (C). Circulating cancer cells and EVs from cancer cells, platelets, and ECs are strongly associated with cancer progression, including metastasis. EC, endothelial cell; EV, extracellular vesicle; VEGF, vascular endothelia growth factor; FGF, fibroblast growth factor; MV, microvesicle; EPC, endothelial progenitor cell.