Fig. 4.
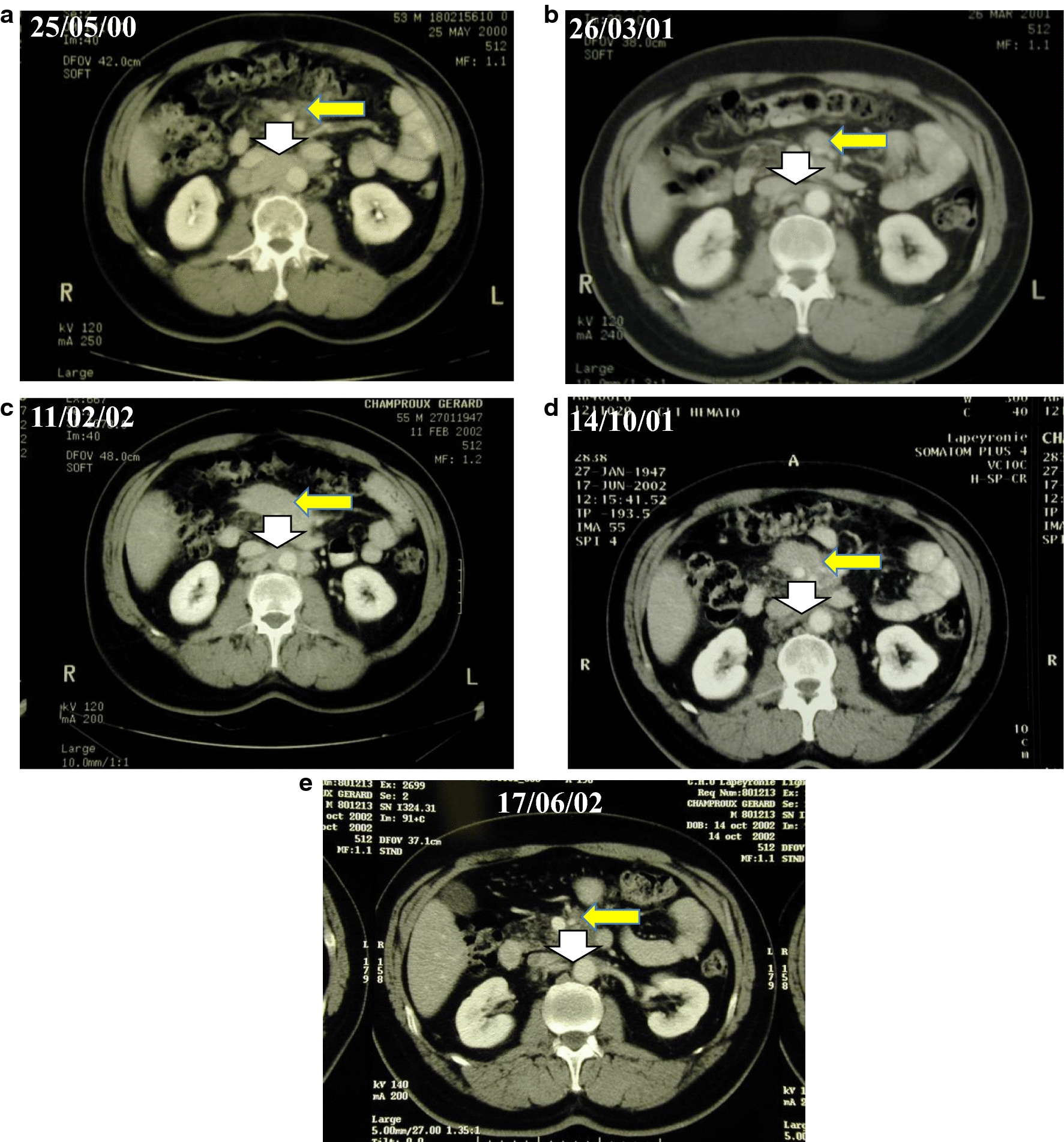
Delayed complete response observed in a patient receiving GM-CSF and rituximab, suggesting an in vivo vaccination. A 58 years-old Male was diagnosed in December 1996 as follicular lymphoma grade II, stage IIIAb, FLIPI 2 with high tumor burden. He received mini-CHOP and interferon alfa followed by a partial response. His disease progressed in March 1998 and he was treated by three courses of DHAP and high dose therapy with autologous transplantation associated with a complete response. He relapsed in May 1999 and received rituximab with partial response. After reprogression in May 2000 (a), he received GM-CSF and rituximab with a long-lasting regression and fluctuation of the tumor over 2 years, as observed on the following scanners (b–d) and no tumor on e, suggesting an in vivo vaccination. He reprogressed in October 2006 and was treated by rituximab with stabilization for 1 year. FLIPI: Follicular Lymphoma International Prognostic Index; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; DHAP: dexamethasone, cytarabine, cis-platinum; GM-CSF: Granulocyte–Macrophage Colony Stimulating Factor
